Prevnar
Generic name: pneumococcal 7-valent vaccine
Dosage form: injection, suspension
Drug class: Bacterial vaccines
Medically reviewed by A Ras MD.
What is Prevnar used for?
Prevnar is a prescription medicine that is used to prevent infections caused by Streptococcus pneumoniae.
Description
Prevnar 13, Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein) is a sterile suspension of saccharides of the capsular antigens of Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, individually linked to non-toxic diphtheria CRM197 protein. Each serotype is grown in soy peptone broth. The individual polysaccharides are purified through centrifugation, precipitation, ultrafiltration, and column chromatography.
The polysaccharides are chemically activated to make saccharides, which are directly conjugated by reductive amination to the protein carrier CRM197, to form the glycoconjugate. CRM197 is a nontoxic variant of diphtheria toxin isolated from cultures of Corynebacterium diphtheriae strain C7 (β197) grown in a casamino acids and yeast extract-based medium or in a chemically-defined medium. CRM197 is purified through ultrafiltration, ammonium sulfate precipitation, and ion-exchange chromatography. The individual glycoconjugates are purified by ultrafiltration and column chromatography and analyzed for saccharide to protein ratios, molecular size, free saccharide, and free protein.
The individual glycoconjugates are compounded to formulate Prevnar 13. Potency of the formulated vaccine is determined by quantification of each of the saccharide antigens and by the saccharide to protein ratios in the individual glycoconjugates. Each 0.5 mL dose of the vaccine is formulated to contain approximately 2.2 µg of each of Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 23F saccharides, 4.4 μg of 6B saccharides, 34 µg CRM197 carrier protein, 100 µg polysorbate 80, 295 µg succinate buffer and 125 µg aluminum as aluminum phosphate adjuvant.
The tip cap and rubber plunger of the prefilled syringe are not made with natural rubber latex.
Mechanism of Action
Prevnar 13, comprised of pneumococcal polysaccharides conjugated to a carrier protein (CRM197), elicits a T-cell dependent immune response. Protein carrier-specific T-cells provide the signals needed for maturation of the B-cell response.
Nonclinical and clinical data support opsonophagocytic activity, as measured by opsonophagocytic activity (OPA) antibody assay, as a contributor to protection against pneumococcal disease. The OPA antibody assay provides an in vitro measurement of the ability of serum antibodies to eliminate pneumococci by promoting complement-mediated phagocytosis and is believed to reflect relevant in vivo mechanisms of protection against pneumococcal disease. OPA antibody titers are expressed as the reciprocal of the highest serum dilution that reduces survival of the pneumococci by at least 50%.
In infants that have received Prevnar 13, opsonophagocytic activity correlates well with serotype specific anti-capsular polysaccharide IgG levels as measured by ELISA. A serum anti-capsular polysaccharide antibody concentration of 0.35 µg/mL as measured by ELISA one month after the third dose as a single antibody reference concentration was used to estimate the effectiveness of Prevnar 13 against invasive pneumococcal disease (IPD) in infants and children.
The assay used for this determination is a standardized ELISA involving pre-absorption of the test sera with pneumococcal C-polysaccharide and serotype 22F polysaccharide to reduce non-specific background reactivity. The single antibody reference value was based on pooled efficacy estimates from three placebo-controlled IPD efficacy trials with either Prevnar or the investigational 9-valent CRM197 conjugate pneumococcal polysaccharide vaccine. This reference concentration is only applicable on a population basis and cannot be used to predict protection against IPD on an individual basis. Functional antibodies elicited by the vaccine (as measured by a dribble opsonophagocytic activity [dOPA] antibody assay) were also evaluated in infants.
In adults, an antipolysaccharide binding antibody IgG level to predict protection against invasive pneumococcal disease or non-bacteremic pneumonia has not been defined. Noninferiority trials for Prevnar 13 were designed to show that functional OPA antibody responses (as measured by a microcolony OPA [mcOPA] antibody assay) for the Prevnar 13 serotypes are noninferior and for some serotypes superior to the common serotypes in the currently licensed pneumococcal polysaccharide vaccine (PPSV23). OPA antibody titers measured in the mcOPA antibody assay cannot be compared directly to titers measured in the dOPA antibody assay.
Before taking Prevnar, tell your doctor:
- If your child has an allergy to any part of Prevnar.
- If your child is allergic to Prevnar; any part of this medicine; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had.
- If your child has low platelet levels.
- If your child has bleeding problems.
- If your child has an infection or an illness with a fever.
If your child is pregnant or breast-feeding a baby:
- Do not give Prevnar to your child if she is pregnant.
- Be sure your child does not breast-feed a baby while taking Prevnar.
This is not a list of all drugs or health problems that interact with this medicine.
Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for your child to take Prevnar with all of his/her drugs and health problems. Do not start, stop, or change the dose of any drug your child takes without checking with the doctor.
What are some things I need to know or do while I take Prevnar?
- Tell all of your child’s health care providers that your child is taking Prevnar. This includes your child’s doctors, nurses, pharmacists, and dentists.
- This medicine may not protect all people who use it. Talk with the doctor.
- If your child was born premature, talk with the doctor. Trouble breathing has happened in these children after getting some vaccines.
- Other drugs may be given before Prevnar to help avoid side effects.
- This medicine is not approved for use in children older than 10 years of age or in adults. Talk with the doctor.
How is Prevnar best taken?
Give Prevnar as ordered by your child’s doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into the muscle.
- Your child’s doctor will give Prevnar.
What do I do if I miss a dose?
- Call your child’s doctor to find out what to do.
What are the side effects of Prevnar that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your child’s doctor or get medical help right away if your child has any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Shortness of breath.
What are some other side effects of Prevnar?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your child’s doctor or get medical help if any of these side effects or any other side effects bother your child or do not go away:
- Mild fever.
- Feeling sleepy.
- Not hungry.
- Throwing up.
- Diarrhea.
- Restlessness.
- Feeling irritable.
- Crying that is not normal.
- Trouble moving where the shot was given.
- Pain where the shot was given.
- Redness or swelling where the shot is given.
These are not all of the side effects that may occur. If you have questions about side effects, call your child’s doctor. Call your child’s doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Prevnar?
- If you need to store Prevnar at home, talk with your child’s doctor, nurse, or pharmacist about how to store it.
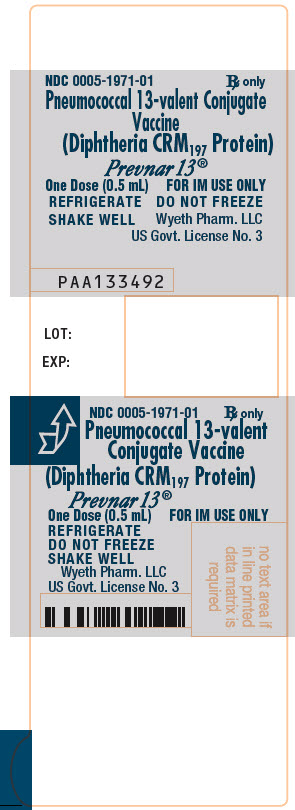
Label
PRINCIPAL DISPLAY PANEL – 0.5 ML SYRINGE LABEL
- NDC 0005-1971-01
Rx only - Pneumococcal 13-valent Conjugate
Vaccine
(Diphtheria CRM197 Protein) - Prevnar 13®
- One Dose (0.5 mL)
FOR IM USE ONLY - REFRIGERATE
DO NOT FREEZE
SHAKE WELL - Wyeth Pharm. LLC
US Govt. License No. 3 - PAA133492
- LOT:
- EXP:
PRINCIPAL DISPLAY PANEL – 10 SYRINGE CARTON
- NDC 0005-1971-02
Rx only - Pneumococcal 13-valent Conjugate Vaccine
(Diphtheria CRM197 Protein) - Prevnar 13®
- 10 One-Dose (0.5 mL)
- Disposable Syringes
- FOR INTRAMUSCULAR USE ONLY
- Pfizer

PRINCIPAL DISPLAY PANEL – 1 SYRINGE CARTON
- NDC 0005-1971-05
- Rx only
- Pneumococcal 13-valent Conjugate Vaccine
(Diphtheria CRM197 Protein) - Prevnar 13®
- One Dose (0.5 mL)
Disposable Syringe - FOR INTRAMUSCULAR USE ONLY
- Pfizer

SRC: NLM .
