Pegvisomant
Generic name: pegvisomant
Brand name: Somavert
Dosage form: subcutaneous powder for injection (10 mg; 15 mg; 20 mg; 25 mg; 30 mg)
Drug class: Growth hormone receptor blockers
Medically reviewed by A Ras MD.
What is pegvisomant used for?
Pegvisomant is a prescription medicine that is used to treat acromegaly.
Description
Pegvisomant is an analog of human growth hormone (GH) of recombinant DNA origin that acts as a GH receptor antagonist.
It contains 191 amino acid residues. The molecular weight of pegvisomant is 22 kDa. The molecular weight of the PEG portion of pegvisomant is approximately 5 kDa. The predominant molecular weights of pegvisomant are thus approximately 42, 47, and 52 kDa. The schematic shows the amino acid sequence of the pegvisomant protein (PEG polymers are shown attached to the 5 most probable attachment sites). Pegvisomant is synthesized by a specific strain of Escherichia coli bacteria that has been genetically modified by the addition of a plasmid that carries a gene for GH receptor antagonist.
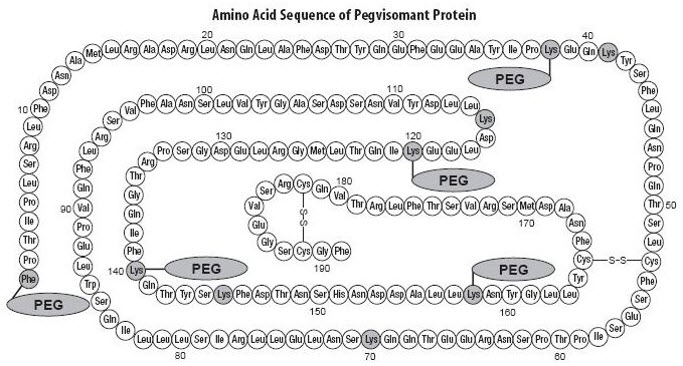 |
| Stippled residues indicate PEG attachment sites (Phe1, Lys38, Lys41, Lys70, Lys115, Lys120, Lys140, Lys145, Lys158) |
Shown below are the amino acid substitutions in pegvisomant, relative to human GH.
| hGH | Pegvisomant |
|---|---|
| His18 | Asp18 |
| Ala21 | Asn21 |
| Gly120 | Lys120 |
| Arg167 | Asn167 |
| Lys168 | Ala168 |
| Asp171 | Ser171 |
| Lys172 | Arg172 |
| Glu174 | Ser174 |
| Ile179 | Thr179 |
SOMAVERT (pegvisomant) for injection is a sterile, white lyophilized powder intended for subcutaneous injection after reconstitution. SOMAVERT is supplied in packages that include a single-dose prefilled syringe containing 1 mL of Sterile Water for Injection, USP, that is a sterile, nonpyrogenic preparation of water for injection that contains no bacteriostat, antimicrobial agent, or added buffer, to be used as a diluent.
SOMAVERT is available in single-dose sterile vials containing 10 mg, 15 mg, 20 mg, 25 mg or 30 mg of pegvisomant. SOMAVERT 10 mg, 15 mg, and 20 mg vials also contain glycine (1.36 mg), mannitol (36 mg), sodium dihydrogen phosphate monohydrate (0.36 mg), and sodium phosphate dibasic anhydrous (1.04 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 10 mg/mL, 15 mg/mL and 20 mg/mL, respectively, with a pH of 7.1 – 7.7.
SOMAVERT 25 mg vial also contains glycine (1.7 mg), mannitol (45 mg), sodium dihydrogen phosphate monohydrate (0.45 mg), and sodium phosphate dibasic anhydrous (1.3 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 25 mg/mL with a pH of 7.1 – 7.7.
SOMAVERT 30 mg vial also contains glycine (2.04 mg), mannitol (54 mg), sodium dihydrogen phosphate monohydrate (0.54 mg), and sodium phosphate dibasic anhydrous (1.56 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 30 mg/mL with a pH of 7.1 – 7.7.
Mechanism of Action
Pegvisomant selectively binds to growth hormone (GH) receptors on cell surfaces, where it blocks the binding of endogenous GH, and thus interferes with GH signal transduction.
Inhibition of GH action results in decreased serum concentrations of IGF-I, as well as other GH-responsive serum proteins such as free IGF-I, the acid-labile subunit of IGF-I (ALS), and insulin-like growth factor binding protein-3 (IGFBP-3).
Before taking pegvisomant, tell your doctor:
- If you are allergic to pegvisomant; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take pegvisomant with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take pegvisomant?
- Tell all of your health care providers that you take pegvisomant. This includes your doctors, nurses, pharmacists, and dentists.
- If you have high blood sugar (diabetes), talk with your doctor. This medicine may lower blood sugar. High blood sugar drugs may need to be changed.
- Check your blood sugar as you have been told by your doctor.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- High blood pressure has happened with pegvisomant. Have your blood pressure checked as you have been told by your doctor.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take pegvisomant.
- In women who have fertility problems from acromegaly, pegvisomant may improve fertility. This could lead to pregnancy. If you want to avoid pregnancy, use birth control while taking pegvisomant.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is pegvisomant best taken?
Use pegvisomant as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into the fatty part of the skin in the upper arm, thigh, buttocks, or stomach area.
- If you will be giving yourself the shot, your doctor or nurse will teach you how to give the shot.
- Wash your hands before and after use.
- Take pegvisomant out of the refrigerator and let it come to room temperature before you use it. Be sure you know how long to leave it at room temperature. Do not heat or microwave.
- This medicine needs to be mixed before use. Follow how to mix as you were told by the doctor.
- Do not shake.
- Move the site where you give the shot with each shot.
- Do not use if the solution is cloudy, leaking, or has particles.
- Do not use if the solution foams.
- Do not use if solution changes color.
- Do not give into skin that is irritated, bruised, red, infected, or scarred.
- Do not give into skin within 2 inches of the belly button.
- Throw away needles in a needle/sharp disposal box. Do not reuse needles or other items. When the box is full, follow all local rules for getting rid of it. Talk with a doctor or pharmacist if you have any questions.
- Keep taking pegvisomant as you have been told by your doctor or other health care provider, even if you feel well.
What do I do if I miss a dose?
- Skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of pegvisomant that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of liver problems like dark urine, feeling tired, not hungry, upset stomach or stomach pain, light-colored stools, throwing up, or yellow skin or eyes.
- Signs of high blood pressure like very bad headache or dizziness, passing out, or change in eyesight.
- Chest pain.
- Dizziness or passing out.
- Any unexplained bruising or bleeding.
- Swelling in the arms or legs.
- Change in skin to hard and thick.
What are some other side effects of pegvisomant?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Diarrhea.
- Irritation where the shot is given.
- Back pain.
- Upset stomach.
- Flu-like signs.
- Signs of a common cold.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out pegvisomant?
- Store unopened vials in a refrigerator. Do not freeze.
- Once mixed, use right away. You may keep at room temperature for up to 6 hours.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Lab
PRINCIPAL DISPLAY PANEL – 15 MG VIAL LABEL
- Pfizer
NDC 0009-5177-02 - Somavert®
(pegvisomant) for injection - 15 mg/vial
- For Subcutaneous Use Only
- One Single-Dose Vial
Discard unused portion. - Rx only
- PAA169992

SRC: NLM .
