SYFOVRE
Generic Name: pegcetacoplan injection, for intravitreal use
Brand Name: Syfovre
Drug Class: Monoclonal Antibodies
Updated Feb 28, 2023
Patient Guide
Advise patients that following SYFOVRE administration, patients are at risk of developing neovascular AMD, endophthalmitis, and retinal detachments. If the eye becomes red, sensitive to light, painful, or if a patient develops any change in vision such as flashing lights, blurred vision or metamorphopsia, instruct the patient to seek immediate care from an ophthalmologist [see WARNINGS AND PRECAUTIONS (5.1, 5.2)].
Patients may experience temporary visual disturbances associated either with the intravitreal injection with SYFOVRE or the eye examination [see ADVERSE REACTIONS (6.1)]. Advise patients not to drive or use machinery until visual function has recovered sufficiently.
DESCRIPTION
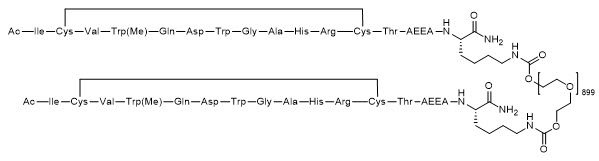
SYFOVRE contains pegcetacoplan, a complement inhibitor. Pegcetacoplan is a symmetrical molecule composed of two identical pentadecapeptides covalently bound to the ends of a linear 40 kiloDalton (kDa) polyethylene glycol (PEG) molecule. The peptide portions of pegcetacoplan contain 1-methyl-L-tryptophan (Trp(Me)) in position 4 and amino (ethoxyethoxy) acetic acid (AEEA) in position 14.
The molecular weight of pegcetacoplan is approximately 43.5 kDa. The molecular formula is C1970H3848N50O947S4. The structure of pegcetacoplan is shown below.
SYFOVRE (pegcetacoplan injection) is a sterile, clear, colorless to light yellow aqueous solution in a single-dose vial for intravitreal use. Each vial allows for the delivery of 0.1 mL of solution containing 15 mg pegcetacoplan, trehalose dihydrate (5.95 mg), glacial acetic acid (0.0895 mg), sodium acetate trihydrate (0.0353 mg), and Water for Injection. SYFOVRE may also contain sodium hydroxide and/or additional glacial acetic acid for adjustment to a target pH of 5.0. SYFOVRE does not contain an anti-microbial preservative.
INDICATIONS AND USAGE
SYFOVRE is indicated for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
DOSAGE AND ADMINISTRATION
General Dosing Information
SYFOVRE must be administered by a qualified physician.
Recommended Dosage
The recommended dose for SYFOVRE is 15 mg (0.1 mL of 150 mg/mL solution) administered by intravitreal injection to each affected eye once every 25 to 60 days.
Preparation for Administration
 |
Store SYFOVRE in the refrigerator between 2°C to 8°C (36°F to 46°F); Keep the vial in the original carton to protect from light. |
 |
Remove the carton from the refrigerator. Keep the vial in the original carton at room temperature 20°C to 25°C (68°F to 77°F), for at least 15 minutes prior to injection, but no longer than 8 hours. Fill the syringe immediately prior to the injection. |
 |
Do not shake the vial. The vial is for use in a single eye. |
 |
Inspect the solution. It is a clear, colorless to light yellow aqueous solution. Do not use if:
|
| STEP 1 Gather the supplies needed: |
|||
|
|||
| Use aseptic technique to carry out the following preparation steps: | |||
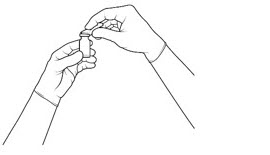
| STEP 2 Remove the flip-off cap from the vial (see FIGURE 1A) and clean the vial septum with an alcohol swab and wait for the alcohol to dry out (see FIGURE 1B). |
|||
|
Figure 1a: |
Figure 1b: |
||
 |
 |
||
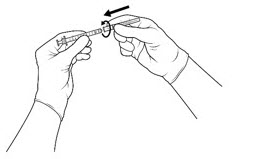
| STEP 3 Attach the 5-micron filter needle onto a 1-mL Luer-lock syringe (see FIGURE 2) by twisting it onto the Luer-lock syringe tip. |
|||
|
Figure 2: |
|||
 |
|||
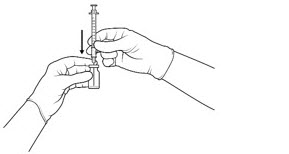
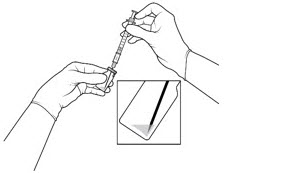
| STEP 4 Push the filter needle into the center of the vial septum until the needle is submerged in the drug product to prevent withdrawal of air (see FIGURE 3A). To withdraw the entire contents of the vial into the syringe, hold the vial at a slightly inclined position. Withdraw the drug product slowly to prevent air bubbles. Continue to tilt the vial during withdrawal keeping the bevel of the filter needle submerged in the liquid until all of the fluid is withdrawn from the vial (see FIGURE 3B). *Do not tap the syringe to remove air bubbles. While maintaining the filter needle within the vial, invert the syringe and move the plunger down and up until bubbles move to the top (see FIGURE 3C). |
|||
|
Figure 3a: |
Figure 3b: |
||
 |
 |
||
|
Figure 3c: |
|||
|
|
|||
| STEP 5 Using aseptic technique, disconnect the filter needle from the syringe and dispose of it. Do not use the filter needle for injection. |
|||
| STEP 6 Aseptically and firmly attach the injection needle onto the 1-mL Luer-lock syringe (see FIGURE 4). |
|||
|
Figure 4: |
|||
 |
|||
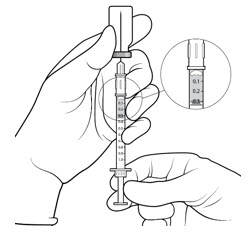
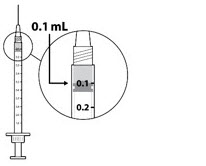
| STEP 7 Check for air bubbles by holding the syringe with the needle pointing up. *Do not tap the syringe to remove air bubbles. If there are any air bubbles, remove the needle cap and with the needle end facing up gently advance the plunger to the 0.1 mL dose mark (see FIGURE 5). Only 0.1 mL (15 mg of SYFOVRE) should be administered to deliver a single dose. Any excess volume should be disposed. The syringe is ready for injection. |
|||
|
Figure 5: |
|||
 |
|||
Injection Procedure
Only 0.1 mL (15 mg of SYFOVRE) should be administered to deliver a single dose. Any excess volume should be disposed. Ensure that the injection is given immediately after the preparation of the dose.
Prior to the intravitreal injection, patients should be monitored for elevated intraocular pressure (IOP) using tonometry [see WARNINGS AND PRECAUTIONS (5.4)]. If necessary, ocular hypotensive medication can be given to lower the IOP. The intravitreal injection procedure should be carried out under aseptic conditions, which includes the use of surgical hand disinfection, sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum topical microbicide to disinfect the periocular skin, eyelid, and ocular surface should be administered prior to the injection.
Inject slowly until the rubber stopper reaches the end of the syringe to deliver the volume of 0.1 mL. Confirm delivery of the full dose by checking that the rubber stopper has reached the end of the syringe barrel.
Immediately following the intravitreal injection, patients should be monitored for elevations in IOP. Additional evaluation may include checking for perfusion of the optic nerve head and tonometry.
Following intravitreal injection patients should be instructed to report any symptoms suggestive of endophthalmitis (e.g., eye pain, redness of the eye, photophobia, blurring of vision) without delay [see PATIENT COUNSELING INFORMATION (17)].
Each vial should only be used for the treatment of a single eye. If the fellow eye requires treatment, a new sterile, vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter, and injection needles should be changed before SYFOVRE administration. Repeat same procedure steps as above.
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
DOSAGE FORMS AND STRENGTHS
Injection: 150 mg/mL, clear, colorless to light yellow aqueous solution in a single-dose vial for administration of a single 0.1 mL dose.
CONTRAINDICATIONS
Ocular or Periocular Infections
SYFOVRE is contraindicated in patients with ocular or periocular infections [see WARNINGS AND PRECAUTIONS (5.1)].
Active Intraocular Inflammation
SYFOVRE is contraindicated in patients with active intraocular inflammation.
WARNINGS AND PRECAUTIONS
Endophthalmitis and Retinal Detachments
Intravitreal injections, including those with SYFOVRE, may be associated with endophthalmitis and retinal detachments [see ADVERSE REACTIONS (6.1)]. Proper aseptic injection technique must always be used when administering SYFOVRE in order to minimize the risk of endophthalmitis [see DOSAGE AND ADMINISTRATION (2.4)]. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately.
Neovascular AMD
In clinical trials, use of SYFOVRE was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (12% when administered monthly, 7% when administered every other month and 3% in the control group ) by Month 24. Patients receiving SYFOVRE should be monitored for signs of neovascular AMD. In case anti-Vascular Endothelial Growth Factor (anti-VEGF) is required, it should be given separately from SYFOVRE administration.
Intraocular Inflammation
In clinical trials, use of SYFOVRE was associated with episodes of intraocular inflammation including: vitritis, vitreal cells, iridocyclitis, uveitis, anterior chamber cells, iritis, and anterior chamber flare. After inflammation resolves patients may resume treatment with SYFOVRE.
Increased Intraocular Pressure
Acute increase in IOP may occur within minutes of any intravitreal injection, including with SYFOVRE. Perfusion of the optic nerve head should be monitored following the injection and managed as needed [see DOSAGE AND ADMINISTRATION (2.4)].
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Ocular and periocular infections [see CONTRAINDICATIONS (4.1)]
- Active intraocular inflammation [see CONTRAINDICATIONS (4.2)]
- Endophthalmitis and retinal detachments [see WARNINGS AND PRECAUTIONS (5.1)]
- Neovascular AMD [see WARNINGS AND PRECAUTIONS (5.2)]
- Intraocular inflammation [see WARNINGS AND PRECAUTIONS (5.3)]
- Increased intraocular pressure [see WARNINGS AND PRECAUTIONS (5.4)]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 839 patients with GA in two Phase 3 studies (OAKS and DERBY) were treated with intravitreal SYFOVRE, 15 mg (0.1 mL of 150 mg/mL solution). Four hundred nineteen (419) of these patients were treated in the affected eye monthly and 420 were treated in the affected eye every other month. Four hundred seventeen (417) patients were assigned to sham.
The most common adverse reactions (≥5%) reported in patients receiving SYFOVRE were ocular discomfort, neovascular age-related macular degeneration, vitreous floaters, and conjunctival hemorrhage.
| Adverse Reactions | PM (N = 419) % |
PEOM (N = 420) % |
Sham Pooled (N = 417) % |
|---|---|---|---|
| PM: SYFOVRE monthly; PEOM: SYFOVRE every other month | |||
|
|||
| Ocular discomfort* | 13 | 10 | 11 |
| Neovascular age-related macular degeneration* | 12 | 7 | 3 |
| Vitreous floaters | 10 | 7 | 1 |
| Conjunctival hemorrhage | 8 | 8 | 4 |
| Vitreous detachment | 4 | 6 | 3 |
| Retinal hemorrhage | 4 | 5 | 3 |
| Punctate keratitis* | 5 | 3 | <1 |
| Posterior capsule opacification | 4 | 4 | 3 |
| Intraocular inflammation* | 4 | 2 | <1 |
| Intraocular pressure increased | 2 | 3 | <1 |
Endophthalmitis, retinal detachment, hyphema and retinal tears were reported in less than 1% of patients.
Optic ischemic neuropathy was reported in 1.7% of patients treated monthly, 0.2% of patients treated every other month and 0.0% of patients assigned to sham. Deaths were reported in 6.7% of patients treated monthly, 3.6% of patients treated every other month and 3.8% of patients assigned to sham. The rates and causes of death were consistent with the elderly study population.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no adequate and well-controlled studies of SYFOVRE administration in pregnant women to inform a drug-associated risk. The use of SYFOVRE may be considered following an assessment of the risks and benefits.
Systemic exposure of SYFOVRE following ocular administration is low [see CLINICAL PHARMACOLOGY (12.3)]. Subcutaneous administration of pegcetacoplan to pregnant monkeys from the mid gestation period through birth resulted in increased incidences of abortions and stillbirths at systemic exposures 1040-fold higher than that observed in humans at the maximum recommended human ophthalmic dose (MRHOD) of SYFOVRE (based on the area under the curve (AUC) systemically measured levels). No adverse maternal or fetal effects were observed in monkeys at systemic exposures approximately 470-fold higher than that observed in humans at the MRHOD (see DATA).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In embryofetal development studies, subcutaneous administration of pegcetacoplan to pregnant cynomolgus monkeys from the mid gestation period through birth produced increased incidences of abortions and stillbirths at doses of 28 mg/kg/day [approximately 1040-fold higher than the MRHOD based on exposure (AUC)]. Pegcetacoplan was not maternally toxic and did not produce adverse embryofetal effects in the monkey at subcutaneous doses of 7 mg/kg/day. (approximately 470-fold higher than the MRHOD). No developmental effects were observed in infants up to 6 months postpartum. Minimal systemic exposure to pegcetacoplan (less than 1%, not pharmacologically significant) was detected in fetuses from monkeys treated subcutaneously with 28 mg/kg/day from the period of organogenesis through the second trimester.
Lactation
Risk Summary
It is not known whether intravitreal administered pegcetacoplan is secreted in human milk or whether there is potential for absorption and harm to the infant. Animal data suggest that the risk of clinically relevant exposure to the infant following maternal intravitreal treatment is minimal (see DATA). Because many drugs are excreted in human milk, and because the potential for absorption and harm to infant growth and development exists, caution should be exercised when SYFOVRE is administered to a nursing woman.
Data
Animal Data
Pegcetacoplan was detectable in milk of lactating monkeys after subcutaneous administration at less than 1% concentration of serum levels but was not detectable in the serum of nursing infants.
Females and Males of Reproductive Potential
Contraception
Females
It is recommended that women of childbearing potential use effective contraception methods to prevent pregnancy during treatment with intravitreal pegcetacoplan. Advise female patients of reproductive potential to use effective contraception during treatment with SYFOVRE and for 40 days after the last dose. For women planning to become pregnant, the use of SYFOVRE may be considered following an assessment of the risks and benefits.
Pediatric Use
The safety and effectiveness of SYFOVRE in pediatric patients have not been established.
Geriatric Use
In clinical studies, approximately 97% (813/839) of patients randomized to treatment with SYFOVRE were ≥ 65 years of age and approximately 72% (607/839) were ≥ 75 years of age. No significant differences in efficacy or safety were seen with increasing age in these studies. No dosage regimen adjustment is recommended based on age.
CLINICAL PHARMACOLOGY
Mechanism of Action
Pegcetacoplan binds to complement protein C3 and its activation fragment C3b with high affinity thereby regulating the cleavage of C3 and the generation of downstream effectors of complement activation
Pharmacodynamics
Cardiac Electrophysiology
Subcutaneous (SC) dosing of pegcetacoplan did not result in QT prolongation. Systemic exposures following intravitreal (IVT) administration are much lower than with SC administration, therefore QT prolongation following IVT pegcetacoplan is not expected.
Pharmacokinetics
SYFOVRE is administered directly into the vitreous to exert local effects in the eye.
Following repeat intravitreal administration of pegcetacoplan at a dose of 15 mg, geometric mean (%CV) serum Cmax value at steady state was 2.2 μg/mL (28.7%) and 1.5 μg/mL (58.1%) for GA patients dosed monthly and every other month, respectively. The steady state geometric mean (%CV) serum trough concentrations were 1.0 μg/mL (52.3%) and 0.2 μg/mL (89.6%) for patients treated monthly and every other month, respectively.
Absorption
Following intravitreal administration of pegcetacoplan, the systemic median Tmax of pegcetacoplan is between 7 and 14 days. Following intravitreal treatment, systemic exposure of pegcetacoplan increases approximately proportionally over a dosage range from 4 to 20 mg.
Distribution
The geometric mean (95%CI) volume of distribution of pegcetacoplan is approximately 1.85 L (1.62 – 2.12) in patients with GA following intravitreal administration.
Elimination
The estimated geometric mean (%CV) of clearance (CL) is 0.284 L/day (21.1%) and geometric mean half-life of elimination (t1/2) is 4.5 days (21.1%) in patients with GA.
Metabolism
Pegcetacoplan is expected to be metabolized into small peptides and amino acids by catabolic pathways.
Specific Populations
There were no clinically significant differences on the pharmacokinetics of pegcetacoplan intravitreal administration based on age (60 to 97 years old), gender, renal impairment, and hepatic function as evaluated by total bilirubin (0.05-1.7 mg/dL), albumin (2.96-5.38 g/dL), aspartate aminotransferase (8.7-101 IU/L), or alanine aminotransferase (5.9-136 IU/L).
Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Difference in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies.
In the 24-month period in studies OAKS and DERBY, the incidence of anti-pegcetacoplan peptide antibodies in patients treated with pegcetacoplan was 4% (18/415 evaluable patients) and 2.5% (10/404 evaluable patients), respectively. Because of the low incidence of anti-pegcetacoplan peptide antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of pegcetacoplan is unknown.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal carcinogenicity studies of pegcetacoplan have not been conducted.
Mutagenesis
Pegcetacoplan was not mutagenic when tested in in vitro bacterial reverse mutation (Ames) assays and was not genotoxic in an in vitro assay in human TK6 cells or in an in vivo micronucleus assay in mice.
Impairment of Fertility
There are no fertility data in humans or animals. There were no microscopic abnormalities in male or female reproductive organs in toxicity studies in rabbits and monkeys indicative of an impact of pegcetacoplan on fertility.
CLINICAL STUDIES
The safety and efficacy of SYFOVRE were assessed in two multi-center, randomized, sham-controlled studies in patients with GA (atrophic nonexudative age-related macular degeneration), with or without subfoveal involvement, secondary to AMD in a total of 1258 randomized patients (SYFOVRE 839 patients, sham 419 patients). Studies OAKS (APL2-304; NCT03525613) and DERBY (APL2-303; NCT03525600) were 24 months in duration, in which patients received treatment for the entire length of the study.
Patient ages ranged from 60 to 100 years with a mean of 78.7 years. Mean (standard deviation) total area of GA lesions(s) at baseline in the study eye (in mm2) were 8.23 (3.83) and 8.29 (4.11) for OAKS and DERBY respectively.
In each study, patients were randomly assigned in a 2:2:1:1 ratio to 1 of 4 dosing regimens:
- 1) SYFOVRE administered at 15 mg/0.1 mL monthly;
- 2) SYFOVRE administered at 15 mg/0.1 mL every other month;
- 3) sham administered monthly;
- 4) sham administered every other month.
In OAKS, 31% of patients in the monthly group, 21% of patients in the every other month and 25% of the patients assigned to sham discontinued treatment prior to Month 24. In DERBY, 29% of patients in the monthly group, 22% of patients in the every other month and 21% of the patients assigned to sham discontinued treatment prior to Month 24.
There was a reduction in the mean rate of GA lesion growth observed in both studies.
Detailed results are shown in Table 2 and Figures 1 and 2.
| Time Period | Group | Rate of GA Lesion Area Growth (mm2) * | ||
|---|---|---|---|---|
| Slope (SE) | Difference (95% CI) in Slope from Sham Pooled | Percent Difference from Sham Pooled | ||
| GA: Geographic atrophy; FAF: fundus autofluorescence; PM: SYFOVRE monthly; PEOM: SYFOVRE every other month; SE: standard error; CI: confidence interval | ||||
|
||||
| OAKS (PM: N=202; PEOM: N=205; Sham Pooled: N=207) | ||||
| Baseline to Month 24† | PM | 3.11 (0.148) | −0.87 (−1.27 to −0.47) |
−21.9% |
| PEOM | 3.26 (0.134) | −0.72 (−1.10 to −0.33) |
−18.1% | |
| Sham Pooled | 3.98 (0.143) | NA | ||
| Baseline to Month 6 | PM | 0.76 (0.046) | −0.22 (−0.35 to −0.09) |
−22.7% |
| PEOM | 0.82 (0.043) | −0.16 (−0.29 to −0.04) |
−16.4% | |
| Sham Pooled | 0.98 (0.047) | NA | ||
| Month 6 to Month 12 | PM | 0.78 (0.060) | −0.19 (−0.34 to −0.03) |
−19.2% |
| PEOM | 0.82 (0.057) | −0.15 (−0.30 to −0.00) |
−15.7% | |
| Sham Pooled | 0.97 (0.052) | NA | ||
| Month 12 to Month 18 | PM | 0.80 (0.050) | −0.23 (−0.37 to −0.08) |
−22.1% |
| PEOM | 0.87 (0.050) | −0.16 (−0.30 to −0.02) |
−15.4% | |
| Sham Pooled | 1.03 (0.053) | NA | ||
| Month 18 to Month 24 | PM | 0.76 (0.067) | −0.23 (−0.41 to −0.06) |
−23.5% |
| PEOM | 0.75 (0.044) | −0.25 (−0.39 to −0.10) |
−24.7% | |
| Sham Pooled | 1.00 (0.058) | NA | ||
| DERBY (PM: N=201; PEOM: N=201; Sham Pooled: N=195) | ||||
| Baseline to Month 24† | PM | 3.28 (0.125) | −0.73 (−1.14 to −0.31) |
−18.1% |
| PEOM | 3.31 (0.129) | −0.70 (−1.11 to −0.28) |
−17.4% | |
| Sham Pooled | 4.00 (0.169) | NA | ||
| Baseline to Month 6 | PM | 0.91 (0.048) | −0.05 (−0.19 to 0.08) |
−5.7% |
| PEOM | 0.88 (0.047) | −0.08 (−0.21 to 0.05) |
−8.2% | |
| Sham Pooled | 0.96 (0.050) | NA | ||
| Month 6 to Month 12 | PM | 0.84 (0.051) | −0.17 (−0.33 to −0.02) |
−17.0% |
| PEOM | 0.85 (0.051) | −0.17 (−0.32 to −0.01) |
−16.4% | |
| Sham Pooled | 1.01 (0.060) | NA | ||
| Month 12 to Month 18 | PM | 0.90 (0.049) | −0.14 (−0.29 to 0.00) |
−13.8% |
| PEOM | 0.88 (0.051) | −0.17 (−0.32 to −0.02) |
−16.0% | |
| Sham Pooled | 1.05 (0.056) | NA | ||
| Month 18 to Month 24 | PM | 0.63 (0.068) | −0.35 (−0.52 to −0.18) |
−36.1% |
| PEOM | 0.69 (0.053) | −0.28 (−0.43 to −0.14) |
−29.1% | |
| Sham Pooled | 0.98 (0.055) | NA | ||
Figure 1: Mean Rate of Change from Baseline in Study Eye GA Lesion Area Measured by FAF in OAKS
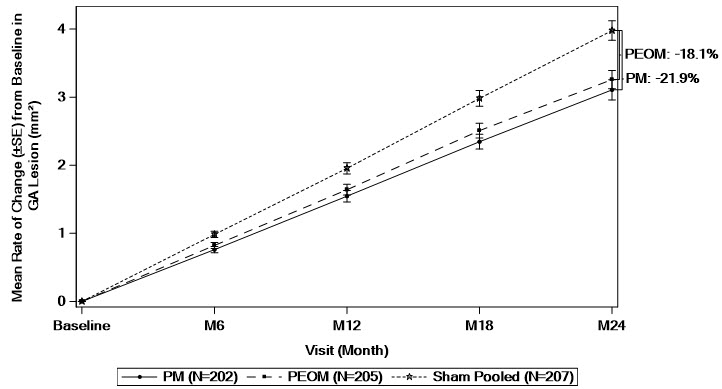
GA: Geographic atrophy; FAF: fundus autofluorescence; PM: SYFOVRE monthly; PEOM: SYFOVRE every other month; SE: standard error.
Based on a mixed effects model for repeated measures assuming a piecewise linear trend in time with knots at Month 6, Month 12, and Month 18. The model included effects for treatment, baseline GA lesion area (<7.5 mm2 or ≥7.5 mm2), time terms, presence of choroidal neovascularization in the fellow eye (yes or no), time terms by treatment interactions, and time terms by baseline GA lesion area (<7.5 mm2 or ≥7.5 mm2) interactions. Time terms include a linear effect of time for each 6-month time period.
Figure 2: Mean Rate of Change from Baseline in Study Eye GA Lesion Area Measured by FAF in DERBY
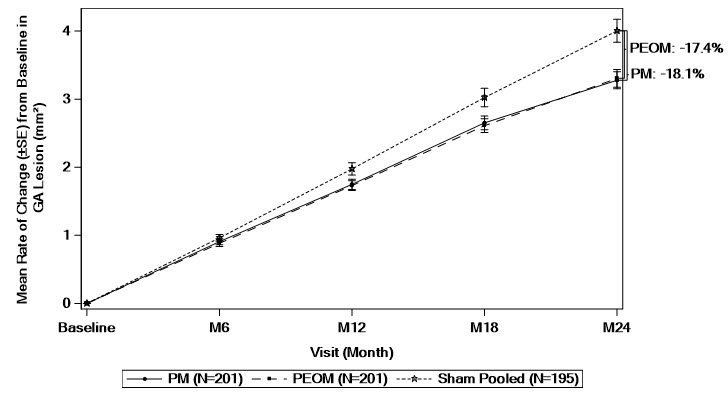
GA: Geographic atrophy; FAF: fundus autofluorescence; PM: SYFOVRE monthly; PEOM: SYFOVRE every other month; SE: standard error.
Based on a mixed effects model for repeated measures assuming a piecewise linear trend in time with knots at Month 6, Month 12, and Month 18. The model included effects for treatment, baseline GA lesion area (<7.5 mm2 or ≥7.5 mm2), time terms, presence of choroidal neovascularization in the fellow eye (yes or no), time terms by treatment interactions, and time terms by baseline GA lesion area (<7.5 mm2 or ≥7.5 mm2) interactions. Time terms include a linear effect of time for each 6-month time period.
CLOSE
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
SYFOVRE (pegcetacoplan injection) is supplied as a clear, colorless to light yellow aqueous solution. Each SYFOVRE carton (NDC 73606-020-01) contains one single-dose glass vial. Each glass vial contains an overfill amount to allow for administration of a single 0.1 mL dose of solution containing 15 mg of SYFOVRE.
Storage and Handling
Refrigerate SYFOVRE between 2°C to 8°C (36°F to 46°F). Store the vial in the original carton to protect from light.
Do not use beyond the expiration date on the carton.
Do not shake.
SPL UNCLASSIFIED SECTION
Manufactured for:
Apellis Pharmaceuticals, Inc.
100 Fifth Avenue
Waltham, MA 02451
For patent information: www.apellis.com/productpatent
Copyright © 2023 Apellis Pharmaceuticals, Inc. All rights reserved.
SYFOVRE is a trademark of Apellis Pharmaceuticals, Inc.
SYF-PI-17Feb2023-1.0
PRINCIPAL DISPLAY PANEL – 15 MG VIAL CARTON
Apellis
NDC 73606-020-01
SYFOVRE™
(pegcetacoplan injection)
15 mg (0.1 mL of 150 mg/mL solution)
One single-dose vial. Discard unused portion.
For Intravitreal Use.
Rx Only