Parsabiv
Generic name: etelcalcetide
What is Parsabiv used for?
Parsabiv is used to treat high parathyroid hormone in patients with kidney disease and patients on dialysis.
Description
PARSABIV (etelcalcetide) is a synthetic peptide calcium-sensing receptor agonist. Etelcalcetide is a white to off-white powder with a molecular formula of C38H73N21O10S2∙xHCl (4 ≤ x ≤ 5) and a molecular weight of 1047.5 g/mol (monoisotopic; free base). It is soluble in water. The hydrochloride salt of etelcalcetide is described chemically as N-acetyl-D-cysteinyl-S-(L-cysteine disulfide)-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide hydrochloride.

PARSABIV (etelcalcetide) injection is supplied in a single-dose vial containing 5 mg/mL of etelcalcetide as a sterile, preservative-free, ready-to-use clear and colorless solution for intravenous injection.
Each PARSABIV single-dose vial contains 2.5 mg etelcalcetide (equivalent to 2.88 mg etelcalcetide as hydrochloride salt) or 5 mg etelcalcetide (equivalent to 5.77 mg etelcalcetide as hydrochloride salt) or 10 mg etelcalcetide (equivalent to 11.54 mg etelcalcetide as hydrochloride salt). PARSABIV single-dose vial is formulated with 0.85% (w/v) sodium chloride, 10 mM succinic acid, and adjusted to pH 3.3 with sodium hydroxide and/or hydrochloric acid.
Before taking Parsabiv, tell your doctor:
- If you are allergic to Parsabiv; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have low calcium levels.
- If you have cancer in the parathyroid gland.
- If you are not on dialysis.
- If you are taking cinacalcet or have taken it in the last 7 days.
- If you are breast-feeding. Do not breast-feed while you take Parsabiv.
This is not a list of all drugs or health problems that interact with this medicine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Parsabiv with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Parsabiv?
- Tell all of your health care providers that you take Parsabiv. This includes your doctors, nurses, pharmacists, and dentists.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using Parsabiv while you are pregnant.
How is Parsabiv best taken?
Use Parsabiv as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into a vein.
What do I do if I miss a dose?
- Skip the missed dose and go back to your normal time.
What are the side effects of Parsabiv that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of low calcium levels like muscle cramps or spasms, numbness and tingling, or seizures.
- Dizziness.
- Very bad belly pain.
- Black, tarry, or bloody stools.
- Throwing up blood or throw up that looks like coffee grounds.
- A type of abnormal heartbeat (prolonged QT interval) can happen with Parsabiv. Call your doctor right away if you have a fast heartbeat, a heartbeat that does not feel normal, or if you pass out.
- Heart failure has happened with Parsabiv, as well as heart failure that has gotten worse in people who already have it. Tell your doctor if you have heart disease. Call your doctor right away if you have shortness of breath, a big weight gain, a heartbeat that is not normal, or swelling in the arms or legs that is new or worse.
What are some other side effects of Parsabiv?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Diarrhea.
- Upset stomach or throwing up.
- Headache.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How supplied/Storage and handling
PARSABIV (etelcalcetide) injection is supplied in a single-dose vial (type I glass) with stopper (fluoropolymer laminated elastomeric) and an aluminum seal with flip-off dust cover containing 5 mg/mL of etelcalcetide as a ready-to-use clear and colorless solution in the following strengths:
| 2.5 mg/0.5 mL | Carton of 10 single-dose vials | NDC 55513-740-10 |
| 5 mg/mL | Carton of 10 single-dose vials | NDC 55513-741-10 |
| 10 mg/2 mL | Carton of 10 single-dose vials | NDC 55513-742-10 |
Storage
Store in the original carton in refrigerator at 2°C to 8°C (36°F to 46°F) to protect from light. Once removed from the refrigerator:
- Do not expose to temperatures above 25°C (77°F).
- Use within 7 days if stored in the original carton.
- Use within 4 hours and do not expose to direct sunlight if removed from the original carton.
Label
10 x 0.5 mL Single-dose Vials
- NDC 55513-740-10
- AMGEN®
- Parsabiv® (etelcalcetide) Injection
- 2.5 mg/ 0.5 mL
- For Intravenous Use after Hemodialysis
Single-dose vial. Discard unused portion
Store at 2°C to 8°C (36°F to 46°F) in the original carton in order to
protect from light.
Sterile, preservative-free solution - Rx Only

PRINCIPAL DISPLAY PANEL – 5 MG/ML VIAL CARTON
- 10 x 1 mL Single-dose Vials
NDC 55513-741-10 - AMGEN®
- Parsabiv® (etelcalcetide) Injection
- 5 mg/mL
- For Intravenous Use after Hemodialysis
Single-dose vial. Discard unused portion
Store at 2°C to 8°C (36°F to 46°F) in the original carton in order to
protect from light.
Sterile, preservative-free solution - Rx Only

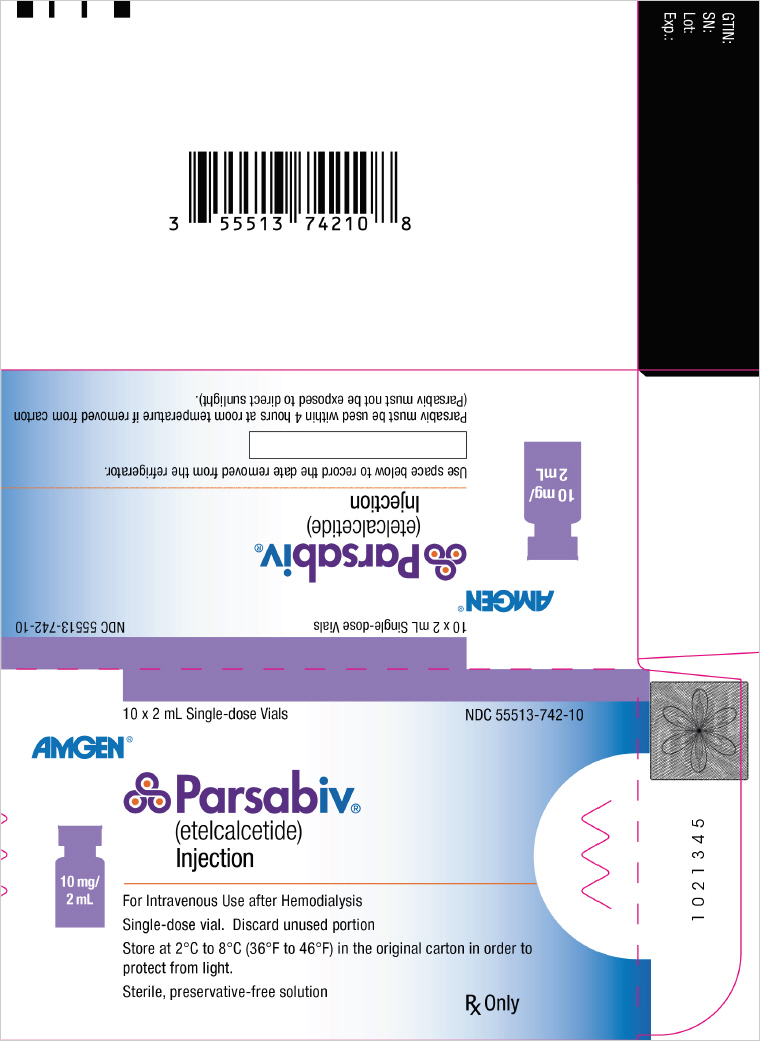
PRINCIPAL DISPLAY PANEL – 10 MG/2 ML VIAL CARTON
- 10 x 2 mL Single-dose Vials
NDC 55513-742-10 - AMGEN®
- Parsabiv® (etelcalcetide) Injection
- 10 mg/ 2 mL
- For Intravenous Use after Hemodialysis
Single-dose vial. Discard unused portion
Store at 2°C to 8°C (36°F to 46°F) in the original carton in order to
protect from light.
Sterile, preservative-free solution - Rx Only