Orbactiv
Generic name: oritavancin
Brand names: Kimyrsa, Orbactiv
Drug class: Glycopeptide antibiotics
Medically reviewed by A Ras MD.
What is Orbactiv used for?
Orbactiv is a prescription medicine that is used to treat skin infections.
Description
ORBACTIV (oritavancin) for injection contains oritavancin diphosphate, a semisynthetic lipoglycopeptide antibacterial drug for intravenous infusion.
The chemical name for oritavancin is [4″R]-22-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-L-arabino-hexopyranosyl)-N3”-[(4′-chloro[1,1′-biphenyl]-4-yl)methyl] vancomycin phosphate [1:2] [salt]. The empirical formula of oritavancin diphosphate is C86H97N10O26Cl3∙2H3PO4 and the molecular weight is 1989.09. The chemical structure is represented below:
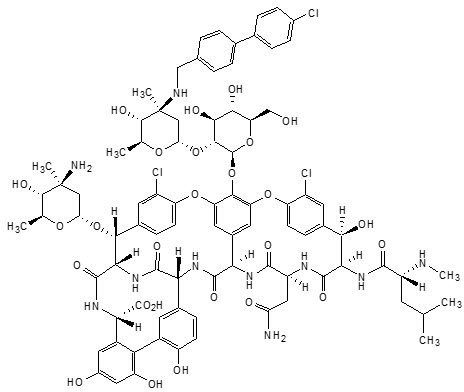
∙2H3PO4
ORBACTIV for injection is supplied as a sterile white to off-white lyophilized powder in a single-dose clear glass vial that contains 400 mg of oritavancin (equivalent to 444 mg oritavancin diphosphate) and the following inactive ingredients: mannitol (200 mg) and phosphoric acid (to adjust pH 3.1 to 4.3).
Each vial is reconstituted with sterile water for injection and further diluted with 5% dextrose injection (D5W) for intravenous infusion. Both the reconstituted solution and the diluted solution for infusion should be a clear, colorless to pale yellow solution, free of visible particles
Before taking Orbactiv, tell your doctor:
- If you are allergic to Orbactiv; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Orbactiv with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Orbactiv?
- Tell all of your health care providers that you take Orbactiv. This includes your doctors, nurses, pharmacists, and dentists.
- Do not use longer than you have been told. A second infection may happen.
- Do not receive heparin through the vein for 120 hours (5 days) after Orbactiv. This medicine may affect certain lab tests used to check how well heparin is working. Talk with your doctor.
- More serious bone infections were seen in people treated with Orbactiv than in people treated with another drug. Talk with your doctor if you have bone pain.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take Orbactiv.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is Orbactiv best taken?
Use Orbactiv as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as an infusion into a vein over a period of time.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of Orbactiv that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs during the infusion like flushing; a rash on the face, neck, trunk, or arms; back pain; chest pain; chills; or shakiness.
- Diarrhea is common with antibiotics. Rarely, a severe form called C diff–associated diarrhea (CDAD) may happen. Sometimes, this has led to a deadly bowel problem (colitis). CDAD may happen during or a few months after taking antibiotics. Call your doctor right away if you have stomach pain, cramps, or very loose, watery, or bloody stools. Check with your doctor before treating diarrhea.
What are some other side effects of Orbactiv?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Headache.
- Irritation where the shot is given.
- Diarrhea, upset stomach, or throwing up.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Orbactiv?
- If you need to store Orbactiv at home, talk with your doctor, nurse, or pharmacist about how to store it.
Label
PRINCIPAL DISPLAY PANEL – 400 MG VIAL CARTON
- Rx only
NDC 70842-140-03 - Orbactiv®
(oritavancin)
for injection - 400 mg per vial
- For Intravenous Infusion Only
- Single-dose vial
Discard Unused Portion - MEL062-R003
- Each vial contains 400 mg of oritavancin
(equivalent to 444 mg oritavancin diphosphate),
200 mg mannitol USP, phosphoric acid for
pH adjustment only. - Recommended Dosage: 1,200 mg administered as
a single dose by intravenous infusion over 3 hours. - Reconstitution: Reconstitute each vial with 40 mL of Sterile Water
for Injection. - Dilution: Reconstituted solution from each vial should be further
diluted in 1000 mL 5% Dextrose Injection ONLY as per instructions
in the prescribing information prior to intravenous infusion.
Do NOT dilute with Sodium Chloride Injection. - Marketed by
Melinta Therapeutics, LLC
Lincolnshire, IL 60069 USA - Contains three 400 mg single-dose vials
SRC: NLM .
