Novolin 70/30
Generic name: insulin isophane and insulin regular
Brand names: HumuLIN 70/30, HumuLIN 70/30 KwikPen, NovoLIN 70/30, ReliOn/NovoLIN 70/30
Drug class: Insulin
Medically reviewed by A Ras MD.
What is Novolin 70/30?
Novolin 70/30 is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Description
NOVOLIN 70/30 (human insulin isophane suspension and human insulin injection) is a mixture of 70% of human insulin isophane, an intermediate-acting human insulin, and 30% of human insulin, a short-acting human insulin. Human insulins are produced by recombinant DNA technology, utilizing Saccharomyces cerevisiae (baker’s yeast) as the production organism. NOVOLIN 70/30 has the empirical formula C257H383N65O77S6 and a molecular weight of 5808.
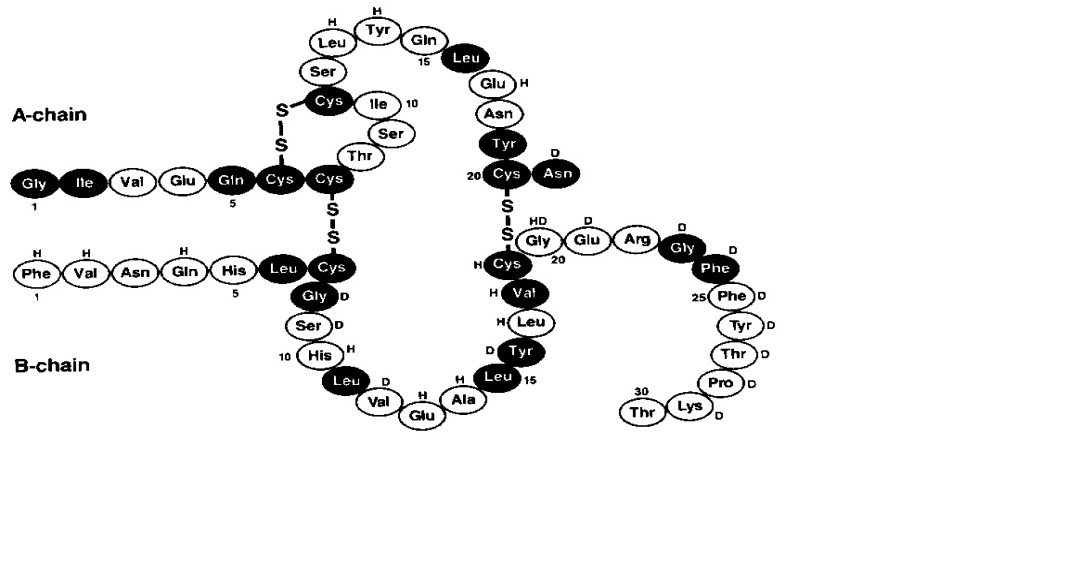
Figure 1: Structural formula of human insulin
NOVOLIN 70/30 is a sterile, white and cloudy suspension that contains human insulin isophane suspension (NPH) and human insulin injection (regular) for subcutaneous use, 100 units/mL, glycerol 16 mg/mL, metacresol 1.5 mg/mL, zinc approximately 20.5 mcg/mL (vial), zinc approximately 30.1 mcg/mL (FlexPen), phenol 0.65 mg/mL, disodium phosphate dihydrate 2.4 mg/mL, protamine sulfate approximately 0.25 mg/mL, and water for injection. The pH is adjusted to 7.1-7.5. Hydrochloric acid 2N and sodium hydroxide 2N are added to adjust pH.
Mechanism of Action
The primary activity of insulin, including NOVOLIN 70/30, is the regulation of glucose metabolism. Insulins lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis, and enhances protein synthesis.
What is the most important information I should know about Novolin 70/30?
Do not share your Novolin 70/30 FlexPen or syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Who should not take Novolin 70/30?
Do not use Novolin 70/30 if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to human insulin isophane, human insulin or any of the ingredients in Novolin 70/30. See the end of this Patient Information guide for a complete list of ingredients in Novolin 70/30.
What should I tell my healthcare provider before taking Novolin 70/30?
Before using Novolin 70/30, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems.
- take other medicines, especially ones called TZDs (thiazolidinediones).
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with Novolin 70/30.
- are pregnant or plan to become pregnant. Talk with your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- are breastfeeding or plan to breastfeed. Novolin 70/30 may pass into your breast milk. Talk with your healthcare provider about the best way to feed your baby while using Novolin 70/30.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
Before you start using Novolin 70/30, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take Novolin 70/30?
- Read the detailed Instructions for Use that comes with your Novolin 70/30.
- Use Novolin 70/30 exactly as your healthcare provider tells you to. Your healthcare provider should tell you how much Novolin 70/30 to use and when to use it.
- Use Novolin 70/30 about 30 minutes before eating a meal.
- Know the type, strength, and amount of insulin you use. Do not change the type, or amount of insulin you use unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you use different types of insulin.
- Check your insulin label each time you give your injection to make sure you are using the correct insulin.
- Inject Novolin 70/30 under the skin (subcutaneously) in your stomach area, buttocks, upper legs (thighs) or upper arms. Do not inject Novolin 70/30 into your vein (intravenously) or muscle (intramuscularly) or use in an insulin infusion pump.
- Do not mix Novolin 70/30 with any other insulins or liquids.
- Change (rotate) your injection site within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
Keep Novolin 70/30 and all medicines out of the reach of children.
Your dose of Novolin 70/30 may need to change because of:
- change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What should I avoid while taking Novolin 70/30?
While using Novolin 70/30 do not:
- drive or operate heavy machinery until you know how Novolin 70/30 affects you.
- drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of Novolin 70/30?
Novolin 70/30 may cause serious side effects that can lead to death, including:
- low blood sugar (hypoglycemia). Signs and symptoms of low blood sugar may include:
- dizziness or lightheadedness, sweating, confusion, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability or mood changes, hunger.
- Your healthcare provider may prescribe a glucagon emergency kit so that others can give you an injection if your blood sugar becomes too low (hypoglycemia) and you are unable to take sugar by mouth.
- dizziness or lightheadedness, sweating, confusion, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability or mood changes, hunger.
- severe allergic reaction (whole body reaction). Get medical help right away if you have any of these signs or symptoms of a severe allergic reaction:
- a rash over your whole body, have trouble breathing, a fast heartbeat, or sweating.
- low potassium in your blood (hypokalemia).
- heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with Novolin 70/30 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Novolin 70/30. Your healthcare provider should monitor you closely while you are taking TZDs with Novolin 70/30. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- shortness of breath, swelling of your ankles or feet, sudden weight gain.Treatment with TZDs and Novolin 70/30 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Get emergency medical help if you have:
- severe hypoglycemia needing hospitalization or emergency room care, and be sure to tell the hospital staff the units of Novolin 70/30 your healthcare provider has prescribed for you.
- trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
The most common side effects of Novolin 70/30 include:
- low blood sugar (hypoglycemia), allergic reactions including reactions at your injection site, skin thickening or pits at the injection site (lipodystrophy), weight gain, swelling (edema) in hands or feet.
These are not all of the possible side effects of Novolin 70/30. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Novolin 70/30
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Novolin 70/30 for a condition for which it was not prescribed. Do not give Novolin 70/30 to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information guide summarizes the most important information about Novolin 70/30. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Novolin 70/30 that is written for healthcare providers. For more information, call 1-800-727-6500 or go to www.novonordisk-us.com.
How should I store Novolin 70/30?
Vials
- Do not freeze Novolin 70/30. Do not use Novolin 70/30 if it has been frozen.
- Keep Novolin 70/30 away from heat or light.
- All unopened vials:
- Store unopened Novolin 70/30 vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unopened vials may be used until the expiration date printed on the label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 42 days, if they are stored at room temperature below 77°F (25°C).
After vials have been opened:
- Opened Novolin 70/30vials can be stored at room temperature below 77°F (25°C). Do not refrigerate.
- Throw away all opened Novolin 70/30vials after 42 days, even if they still have insulin left in them.
FlexPen
- Do not freeze Novolin 70/30. Do not use Novolin 70/30 if it has been frozen.
- Keep Novolin 70/30 away from heat and light.
- Until first use:
- Store unused Novolin 70/30 FlexPen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused Novolin 70/30 FlexPen may be used until the expiration date printed on the label, if kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused Novolin 70/30 FlexPen stored at room temperature should be thrown away after 28 days.
- In-use:
- Store the Novolin 70/30 FlexPen you are currently using out of the refrigerator at room temperature below 86°F (30°C) for up to 28 days.
- The Novolin 70/30 FlexPen you are using should be thrown away after 28 days, even if it still has insulin left in it.
- Store the Novolin 70/30 FlexPen without the needle attached.
Patient Instructions for Use
NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen®
Read the following instructions carefully before you start using your NovoLog® Mix 70/30 FlexPen® and each time you get a refill. There may be new information. You should read the instructions even if you have used Novolog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® before.
NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® is a disposable dial-a-dose insulin pen. You can select doses from 1 to 60 units in increments of 1 unit. NovoLog® Mix 70/30 FlexPen® is designed to be used with NovoFine® needles.
NovoLog Mix® 70/30 FlexPen® should not be used by people who are blind or have severe visual problems without the help of a person who has good eyesight and who is trained to use the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® the right way.
Getting ready
Make sure you have the following items:
- NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen®
- New NovoFine® needle
- Alcohol swab
 |
PREPARING YOUR NOVOLOG® MIX 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FLEXPEN®
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the label to make sure that you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin. NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) should look cloudy after mixing.
Before your first injection with a new NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® you must mix the insulin:
A. Let the insulin reach room temperature before you use it. This makes it easier to mix.
Pull off the pen cap (see diagram A).
 |
B. Roll the pen between your palms 10 times – it is important that the pen is kept horizontal (see diagram B).
 |
C. Then gently move the pen up and down ten times between position 1 and 2 as shown, so the glass ball moves from one end of the cartridge to the other (see diagram C).
 |
Repeat rolling and moving the pen until the liquid appears white and cloudy.
For every following injection move the pen up and down between positions 1 and 2 at least ten times until the liquid appears white and cloudy.
After mixing, complete all the following steps of the injection right away. If there is a delay, the insulin will need to be mixed again.
Wipe the rubber stopper with an alcohol swab.
Before you inject, there must be at least 12 units of insulin left in the cartridge to make sure the remaining insulin is evenly mixed. If there are less than 12 units left, use a new NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® .
Attaching the needle
D. Remove the protective tab from a disposable needle.
 |
Screw the needle tightly onto your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® . It is important that the needle is put on straight (see diagram D).
Never place a disposable needle on your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® until you are ready to take your injection.
E. Pull off the big outer needle cap (see diagram E).
 |
F. Pull off the inner needle cap and dispose of it (see diagram F).
 |
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles.
- Be careful not to bend or damage the needle before use.
- To reduce the risk of a needle stick, never put the inner needle cap back on the needle.
Giving the airshot before each injection:
Before each injection small amounts of air may collect in the cartridge during normal use. To avoid injecting air and to make sure you take the right dose of insulin:
G. Turn the dose selector to select 2 units (see diagram G).
 |
H. Hold your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® with the needle pointing up. Tap the cartridge gently with your finger a few times to make any air bubbles collect at the top of the cartridge (see diagram H).
 |
I. Keep the needle pointing upwards, press the push-button all the way in (see diagram I). The dose selector returns to 0.
 |
A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no more than 6 times.
If you do not see a drop of insulin after 6 times, do not use the NovoLog Mix® 70/30 FlexPen® and contact Novo Nordisk at 1-800-727-6500.
A small air bubble may remain at the needle tip, but it will not be injected.
SELECTING YOUR DOSE
Check and make sure that the dose selector is set at 0.
J. Turn the dose selector to the number of units you need to inject. The pointer should line up with your dose.
 |
The dose can be corrected either up or down by turning the dose selector in either direction until the correct dose lines up with the pointer (see diagram J). When turning the dose selector, be careful not to press the push-button as insulin will come out.
You cannot select a dose larger than the number of units left in the cartridge.
You will hear a click for every single unit dialed. Do not set the dose by counting the number of clicks you hear.
- Do not use the cartridge scale printed on the cartridge to measure your dose of insulin.
GIVING THE INJECTION
Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting. Wipe the skin with an alcohol swab and let the area dry.
K. Insert the needle into your skin.
 |
Inject the dose by pressing the push-button all the way in until the 0 lines up with the pointer (see diagram K). Be careful only to push the button when injecting.
Turning the dose selector will not inject insulin.
L. Keep the needle in the skin for at least 6 seconds, and keep the push-button pressed all the way in until the needle has been pulled out from the skin (see diagram L). This will make sure that the full dose has been given.
 |
You may see a drop of NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) at the needle tip. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with an alcohol swab. Do not rub the area.
After the injection
Do not recap the needle. Recapping can lead to a needle stick injury. Remove the needle from the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® after each injection. This helps to prevent infection, leakage of insulin, and will help to make sure you inject the right dose of insulin.
- Put the needle and any empty NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® or any used NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® still containing insulin in a sharps container or some type of hard plastic or metal container with a screw top such as a detergent bottle or empty coffee can. These containers should be sealed and thrown away the right way. Check with your healthcare provider about the right way to throw away used syringes and needles. There may be local or state laws about how to throw away used needles and syringes. Do not throw away used needles and syringes in household trash or recycling bins.
The NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® prevents the cartridge from being completely emptied. It is designed to deliver 300 units.
M. Put the pen cap on the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® and store the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® without the needle attached (see diagram M).
 |
FUNCTION CHECK
If your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® is not working the right way, follow the steps below:
- Screw on a new NovoFine® needle
- Remove the big outer needle cap and the inner needle cap
- Do an airshot as described in &lquo;Giving the airshot before each injection”.
- Put the big outer needle cap onto the needle. Do not put on the inner needle cap.
- Turn the dose selector so the dose indicator window shows 20 units.
- Hold the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® so the needle is pointing down
- Press the push-button all the way in.
The insulin should fill the lower part of the big outer needle cap (see diagram N). If NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® has released too much or too little insulin, do the function check again. If the same problem happens again, do not use your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® and contact Novo Nordisk at 1-800-727-6500.
 |
Maintenance
Your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® is designed to work accurately and safely. It must be handled with care. Avoid dropping your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® as it may damage it. If you are concerned that your NovoLog® Mix 70/30 FlexPen® is damaged, use a new one. You can clean the outside of your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® by wiping it with a damp cloth. Do not soak or wash your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® as it may damage it. Do not refill your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen®.
- Remove the needle from the NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® after each injection. This helps to ensure sterility, prevent leakage of insulin, and will help to make sure you inject the right dose of insulin for future injections.
- Be careful when handling used needles to avoid needle sticks and transfer of infectious diseases.
- Keep your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® and needles out of the reach of children.
- Use NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® as directed to treat your diabetes. Do not share it with anyone else even if they also have diabetes.
- Always use a new needle for each injection.
- Novo Nordisk is not responsible for harm due to using this insulin pen with products not recommended by Novo Nordisk.
- As a precautionary measure, always carry a spare insulin delivery device in case your NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® is lost or damaged.
- Remember to keep the disposable NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart (rdna origin)) FlexPen® with you. Do not leave it in a car or other location where it can get too hot or too cold.
NovoLog®, FlexPen®, NovoFine®, are trademarks of Novo Nordisk A/S.
NovoLog® is covered by US Patent Nos. 5,547,930, 5,618,913, 5,834,422, 5,840,680, 5,866,538 and other patents pending.
FlexPen® is covered by US Patent Nos. 6,582,404, 6,004,297, 6,235,004 and other patents pending.
© 2002-2010 Novo Nordisk Inc.
What are the ingredients in Novolin 70/30?
Active ingredient: 70% human insulin isophane and 30% human insulin
Inactive ingredients: glycerol, metacresol, zinc, phenol, disodium phosphate dihydrate, protamine sulfate, water for injection, hydrochloric acid and sodium hydroxide.
Label
PRINCIPAL DISPLAY PANEL – 10 ML VIAL RELION
- NDC 0169-1837-02
- Novolin®70/30
- (human insulin isophane suspension and
- human insulin injection)
- 100 units/mL 10 mL multi-dose vial
- ReliOn®

PRINCIPAL DISPLAY PANEL – 3 ML FLEXPEN
- NDC 0169-3007-15
- List: 300715
- Novolin® 70/30
- FlexPen®
- (human insulin isophane suspension
- and human insulin injection)
- 100 units/mL (U-100)
- For Single Patient Use Only
- 5×3 mL Prefilled Pens
- For subcutaneous use only
- Recommended for use with NovoFine®, NovoFine® Plus or NovoTwist®
- disposable needles.
- Until first use: Keep in a cold place.
- Store at 2°C to 8°C (36°F to 46°F).
- Do not freeze.
- In-use: Keep at room temperature below 30°C (86°F) and discard after 28 days.
- Protect from light.
- Dispense in this sealed carton.
- Shake carefully before using.
- See enclosed insert for proper technique.
- 70/30
- novo nordisk®

SRC: NLM .
