Myrbetriq
Generic name: mirabegron
Drug class: Urinary antispasmodics
Medically reviewed by A Ras MD.
What is Myrbetriq?
Adults
- Myrbetriq tablets is a prescription medicine that can be used alone or with solifenacin succinate to treat adults with the following symptoms due to a condition called overactive bladder (OAB):
- Urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- Urgency: a strong need to urinate right away
- Frequency: urinating often
Children
- Myrbetriq tablets is a prescription medicine used to treat children 3 years of age and older weighing at least 77 pounds (35 kg), with a condition called neurogenic detrusor overactivity (NDO).
- Myrbetriq granules is a prescription medicine used to treat children 3 years of age and older with a condition called neurogenic detrusor overactivity (NDO).
It is not known if Myrbetriq tablets and Myrbetriq granules to treat NDO, are safe and effective in children under 3 years of age.
Description
MYRBETRIQ (mirabegron extended-release tablets) for oral use and MYRBETRIQ Granules (mirabegron for extended-release oral suspension) are beta-3 adrenergic agonists.
The chemical name of mirabegron is 2-(2-aminothiazol-4-yl)-N-[4-(2-{[(2R)-2-hydroxy-2-phenylethyl]amino}ethyl)phenyl]acetamide having an empirical formula of C21H24N4O2S and a molecular weight of 396.51. The structural formula of mirabegron is:
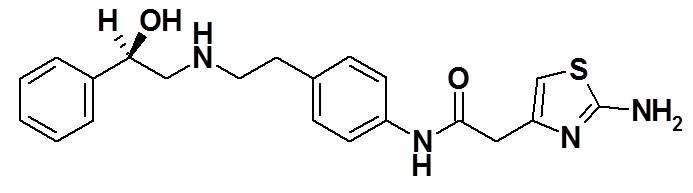
Mirabegron is a white powder. It is practically insoluble in water (0.082 mg/mL). It is soluble in methanol and dimethyl sulfoxide.
Each MYRBETRIQ (mirabegron extended-release tablets) for oral use contains either 25 mg or 50 mg of mirabegron and the following inactive ingredients: butylated hydroxytoluene, hydroxypropyl cellulose, hypromellose, magnesium stearate, polyethylene glycol, polyethylene oxide, red ferric oxide (25 mg tablet only), and yellow ferric oxide.
Each bottle of MYRBETRIQ Granules (mirabegron for extended-release oral suspension) contains approximately 8.3 g of granules, which contain 830 mg of mirabegron, and the following inactive ingredients: acesulfame potassium, diluted hydrochloric acid, ethylparaben, hypromellose, magnesium stearate, mannitol, methylparaben, silicon dioxide, simethicone, sodium polystyrene sulfonate, and xanthan gum. After reconstituted with 100 mL water, the suspension contains 8 mg/mL of mirabegron.
Mechanism of Action
Mirabegron is an agonist of the human beta-3 adrenergic receptor (AR) as demonstrated by in vitro laboratory experiments using the cloned human beta-3 AR. Mirabegron relaxes the detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle by activation of beta-3 AR which increases bladder capacity. Although mirabegron showed very low intrinsic activity for cloned human beta-1 AR and beta-2 AR, results in humans indicate that beta-1 AR stimulation occurred at a mirabegron dose of 200 mg.
Who should not take Myrbetriq?
Do not take Myrbetriq tablets or Myrbetriq granules if you are allergic to mirabegron or any of the ingredients in Myrbetriq tablets or Myrbetriq granules. See the end of this guide for a complete list of ingredients in Myrbetriq tablets or Myrbetriq granules.
What should I tell my healthcare provider before taking Myrbetriq?
Before you take Myrbetriq tablets or Myrbetriq granules, tell your doctor about all of your medical conditions, including if you:
- have liver problems.
- have kidney problems.
- have very high uncontrolled blood pressure.
- have trouble emptying your bladder or you have a weak urine stream.
- are pregnant or plan to become pregnant. It is not known if Myrbetriq tablets or Myrbetriq granules will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Myrbetriq tablets or Myrbetriq granules passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take Myrbetriq tablets or Myrbetriq granules.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Myrbetriq tablets and Myrbetriq granules may affect the way other medicines work, and other medicines may affect how Myrbetriq tablets and Myrbetriq granules work.
Especially tell your doctor if you take:
- thioridazine (Mellaril or Mellaril-S)
- flecainide (Tambocor)
- propafenone (Rythmol)
- digoxin (Lanoxin)
- solifenacin succinate (VESIcare)
How should I take Myrbetriq tablets?
- Take Myrbetriq tablets exactly as your doctor tells you to take it.
- You should take 1 Myrbetriq tablet 1 time a day.
- If your doctor prescribes Myrbetriq tablets and solifenacin succinate together, you should take 1 Myrbetriq tablet and 1 solifenacin succinate tablet at the same time, 1 time a day.
- You should take Myrbetriq tablets with water and swallow the tablet whole.
- Do not chew, break, or crush the tablet.
- Adults can take Myrbetriq tablets with or without food.
- Adults can take Myrbetriq tablets and solifenacin succinate together with or without food.
- Children should take Myrbetriq tablets with food.
- If you miss a dose of Myrbetriq tablets, take it as soon as possible. If it has been more than 12 hours since taking the last dose of Myrbetriq tablets, skip that dose and take the next dose at the usual time.
- If you take too much Myrbetriq tablets, call your doctor or go to the nearest hospital emergency room right away.
How should I take Myrbetriq granules?
- You or your child should take Myrbetriq granules exactly as the doctor tells you to take it.
- You or your child should take Myrbetriq granules by mouth 1 time a day.
- You or your child should take Myrbetriq granules with food.
- You or your child should take Myrbetriq granules immediately after preparation (see the steps below). Do not save the dose for later use.
- If you or your child misses a dose of Myrbetriq granules, take or give it as soon as possible. If it has been more than 12 hours since the last dose of Myrbetriq granules, skip that dose and take or give the next dose at the usual time.
- If you or your child takes too much Myrbetriq granules, call your doctor or go to the nearest hospital emergency room right away.
Note: You will receive Myrbetriq granules from the pharmacy as a suspension. The suspension is prepared by your pharmacist. You will receive an oral dosing device with the suspension. If the suspension will not be used for 2 or more days, shake the bottle vigorously for 1 minute each day to make sure the granules are mixed well (dispersed). Talk to your pharmacist if you have any questions.
Steps to prepare and take the suspension:
Step 1. Shake the bottle vigorously for 1 minute then let it stand until the foam on top of the suspension is gone (approximately 1 to 2 minutes). If the granules have not mixed well (dispersed), shake the bottle vigorously again for 1 minute and let it stand until the foam is gone.
Step 2. Using the oral dosing device provided by the pharmacist, place the dose into the oral dosing device and take the suspension within 1 hour with food. Disregard any bubbles.
Step 3. After each use, wash the oral dosing device with mild household detergent, rinse under running tap water, and allow it to air dry.
What are the possible side effects of Myrbetriq?
Myrbetriq tablets and Myrbetriq granules may cause serious side effects, including:
- increased blood pressure. Myrbetriq tablets and Myrbetriq granules may cause your blood pressure to increase or make your blood pressure worse if you have a history of high blood pressure. You and your doctor should check your blood pressure while you are taking Myrbetriq tablets or Myrbetriq granules. Call your doctor if you have increased blood pressure.
- inability to empty your bladder (urinary retention). Myrbetriq tablets may increase your chances of not being able to empty your bladder if you have bladder outlet obstruction or if you are taking other medicines to treat overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- angioedema. Myrbetriq tablets and Myrbetriq granules may cause an allergic reaction with swelling of the lips, face, tongue, or throat with or without difficulty breathing. Stop using Myrbetriq tablets or Myrbetriq granules and go to the nearest hospital emergency room right away.
Adults with Overactive Bladder
The most common side effects of Myrbetriq tablets include:
- high blood pressure
- pain or swelling of the nose or throat (nasopharyngitis)
- urinary tract infection
- headache
The most common side effects of Myrbetriq tablets, when used with solifenacin succinate, include:
- dry mouth
- urinary tract infection
- constipation
- fast heartbeat
Children with Neurogenic Detrusor Overactivity
The most common side effects of Myrbetriq tablets and Myrbetriq granules include:
- urinary tract infection
- pain or swelling of the nose or throat (nasopharyngitis)
- constipation
- headache
Tell your doctor if you have any side effect that bothers you, does not go away, or if you have swelling of the face, lips, tongue or throat, hives, skin rash or itching while taking Myrbetriq tablets or Myrbetriq granules.
These are not all the possible side effects of Myrbetriq tablets and Myrbetriq granules. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Myrbetriq
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Myrbetriq tablets or Myrbetriq granules for a condition for which it was not prescribed. Do not give Myrbetriq tablets or Myrbetriq granules to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Myrbetriq tablets and Myrbetriq granules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Myrbetriq tablets or Myrbetriq granules that is written for health professionals.
How should I store Myrbetriq?
- Store Myrbetriq tablets at room temperature between 68°F to 77°F (20°C to 25°C). Keep the bottle closed. Throw away (discard) medicine that is out of date or no longer needed.
- Store Myrbetriq granules at room temperature between 68°F to 77°F (20°C to 25°C). Use Myrbetriq granules within 28 days (4 weeks) after the date the pharmacist prepares the suspension. The pharmacist will write the expiration date on the bottle. Throw away (discard) any unused medicine after the expiration date.
Keep Myrbetriq tablets, Myrbetriq granules, and all medicines out of the reach of children.
What are the ingredients in Myrbetriq?
Active ingredient: mirabegron
Inactive ingredients:
Tablets: butylated hydroxytoluene, hydroxypropyl cellulose, hypromellose, magnesium stearate, polyethylene glycol, polyethylene oxide, red ferric oxide (25 mg Myrbetriq tablet only), and yellow ferric oxide.
Granules: acesulfame potassium, diluted hydrochloric acid, ethylparaben, hypromellose, magnesium stearate, mannitol, methylparaben, silicon dioxide, simethicone, sodium polystyrene sulfonate, and xanthan gum.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 25 MG
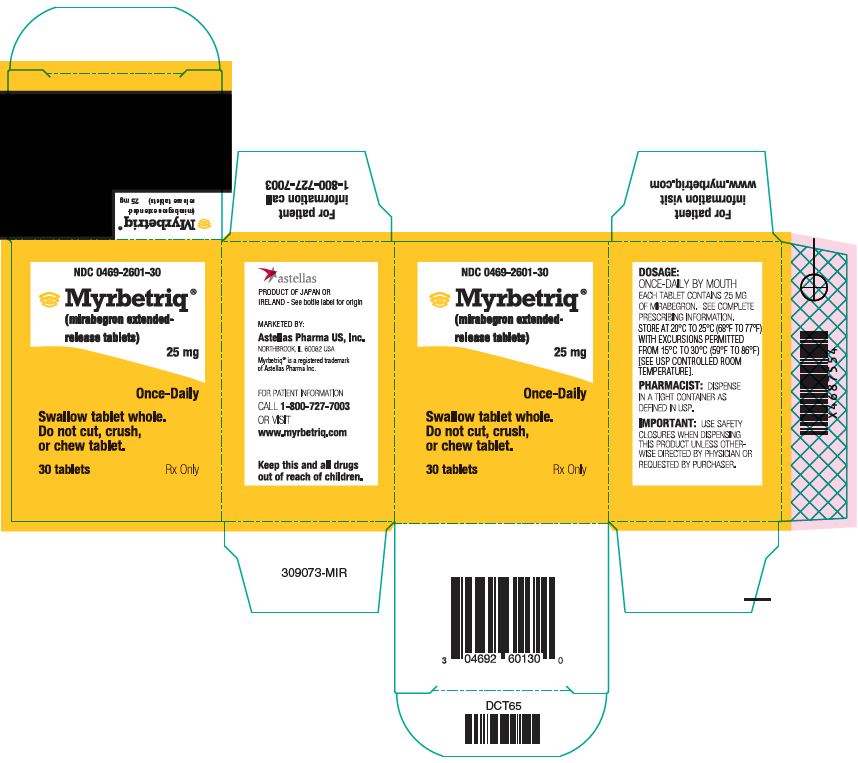

- NDC 0469-2601-30
- Myrbetriq®
(mirabegron extended-release tablets)
25 mg - Once-Daily
- Swallow tablet whole.
Do not cut, crush,
or chew tablet. - 30 tablets
- Rx Only
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 50 MG
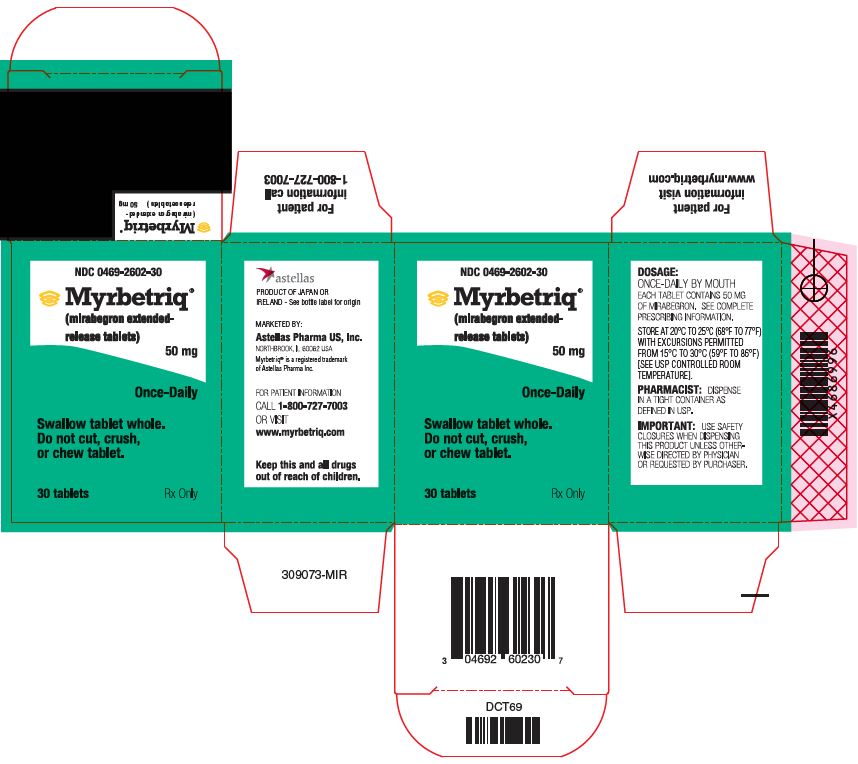

- NDC 0469-2602-30
- Myrbetriq®
(mirabegron extended-release tablets)
50 mg - Once-Daily
- Swallow tablet whole.
Do not cut, crush,
or chew tablet. - 30 tablets
- Rx Only
SRC: NLM .
