Myobloc
Generic name: rimabotulinumtoxinB (Myobloc)
Drug class: Skeletal muscle relaxants
Medically reviewed by A Ras MD.
What is Myobloc?
Myobloc is a prescription medicine used in adults that are injected into muscles and used to treat the abnormal head position and neck pain that happens with cervical dystonia (CD). This medication is also injected into glands that make saliva and is used to treat long-lasting (chronic) drooling (sialorrhea).
It is not known whether Myobloc is safe or effective in children.
Description
RimabotulinumtoxinB is an acetylcholine release inhibitor. RimabotulinumtoxinB is a 700 kDA botulinum toxin type B complex produced from fermentation of the bacterium Clostridium botulinum type B (Bean strain) and exists in noncovalent association with hemagglutinin and nonhemagglutinin proteins as a neurotoxin complex. The neurotoxin complex is recovered from the fermentation process and purified through a series of precipitation and chromatography steps.
MYOBLOC (rimabotulinumtoxinB) injection is a sterile, preservative-free, clear and colorless to light-yellow solution in a single-dose vial for intramuscular or intraglandular use. Each vial contains 2,500 Units/0.5 mL; 5,000 units/mL; or 10,000 Units/2 mL of rimabotulinumtoxinB at a concentration of 5,000 Units/mL at approximately pH 5.6.
Each 2,500 Units/0.5 mL vial of MYOBLOC contains 2,500 Units rimabotulinumtoxinB, 0.235 mg albumin human, 2.9 mg sodium chloride and 1.35 mg sodium succinate.
Each 5,000 Units/mL vial of MYOBLOC contains 5,000 Units rimabotulinumtoxinB, 0.47 mg albumin human, 5.8 mg sodium chloride and 2.7 mg sodium succinate.
Each 10,000 Units/2 mL vial of MYOBLOC contains 10,000 Units rimabotulinumtoxinB, 0.94 mg albumin human, 11.6 mg sodium chloride and 5.4 mg sodium succinate.
One unit of MYOBLOC corresponds to the calculated median lethal intraperitoneal dose (LD50) in mice. The method for performing the assay is specific to Solstice Neurosciences’ manufacture of MYOBLOC. Due to differences in specific details such as the vehicle, dilution scheme and laboratory protocols for various mouse LD50 assays, Units of biological activity of MYOBLOC cannot be compared to or converted into Units of any other botulinum toxin or any toxin assessed with any other specific assay method. Therefore, differences in species sensitivities to different botulinum neurotoxin serotypes preclude extrapolation of animal dose-activity relationships to human dose estimates. The specific activity of MYOBLOC ranges between 70 to 130 Units/ng.
Mechanism of Action
MYOBLOC blocks cholinergic transmission at the neuromuscular and salivary neuroglandular junction by inhibiting the release of acetylcholine from peripheral cholinergic nerved terminals. This inhibition occurs according to the following sequence: neurotoxin binding to cholinergic nerve terminals, internalization of the neurotoxin into the nerve terminal, translocation of the light-chain part of the molecule into the cytosol of the nerve terminal, and enzymatic cleavage of synaptic Vesicle Associated Membrane Protein (VAMP, also known as synaptobrevin), a presynaptic target protein essential for the release of acetylcholine. In both muscles and glands, impulse transmission is re-established by the formation of new nerve endings.
What is the most important information I should know about Myobloc?
Myobloc may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems after treatment with Myobloc:
- Problems swallowing, speaking, or breathing. These problems can happen hours to weeks after an injection of Myobloc if the muscles that you use to breathe and swallow become weak after the injection. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with Myobloc.
- People with certain breathing problems may need to use muscles in their neck to help them breathe. These people may be at greater risk for serious breathing problems with Myobloc.
- Swallowing problems may last for several months. People who cannot swallow well may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving Myobloc have the highest risk of getting these problems.
- Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include:
- loss of strength and muscle weakness all over the body
- double vision
- blurred vision and drooping eyelids
- hoarseness or change or loss of voice (dysphonia)
- trouble saying words clearly (dysarthria)
- loss of bladder control
- trouble breathing
- trouble swallowing (dysphagia)
These symptoms can happen hours to weeks after you receive an injection of Myobloc.
These problems could make it unsafe for you to drive a car or do other dangerous activities. See “What should I avoid while receiving Myobloc?”
Who should not use Myobloc?
Do not receive Myobloc if you:
- are allergic to Myobloc or any of the ingredients in Myobloc. See the end of this Medication Guide for a list of ingredients in Myobloc.
- had an allergic reaction to any other botulinum toxin product such as Botox, Botox Cosmetic (onabotulinumtoxinA), Dysport (abobotulinumtoxinA), or Xeomin (incobotulinumtoxinA).
- have a skin infection at the planned injection site.
What should I tell my healthcare provider before using Myobloc?
Before receiving Myobloc, tell your doctor about all your medical conditions, including if you have:
- a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig’s disease], myasthenia gravis or Lambert-Eaton syndrome). See “What is the most important information I should know about Myobloc?”
- had any side effect from any botulinum toxin product in the past.
- a breathing problem, such as asthma or emphysema.
- a history of swallowing problems or inhaling food or fluid into your lungs (aspiration).
- bleeding problems.
- drooping eyelids.
- plans to have surgery.
- had surgery on your face.
Tell your doctor if you:
- are pregnant or plan to become pregnant. It is not known if Myobloc can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Myobloc passes into breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Talk to your doctor before you take any new medicine after you receive Myobloc.
Using Myobloc with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received Myobloc in the past. Especially tell your doctor if you:
- have received any other botulinum toxin product in the last 4 months.
- have received injections of botulinum toxin such as Botox, Botox Cosmetic (onabotulinumtoxinA), Dysport (abobotulinumtoxinA), or Xeomin incobotulinumtoxinA) in the past. Be sure your doctor knows exactly which product you received.
- have recently received an antibiotic by injection.
- take muscle relaxants.
- take an allergy or cold medicine.
- take a sleep medicine.
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I use Myobloc?
- Myobloc is a shot (injection) that your doctor will give you.
- Myobloc is injected into your affected muscles or glands.
- Your doctor may give you another dose of Myobloc after 12 weeks or longer, if it is needed.
- Your doctor may change your dose of Myobloc, until you and your doctor find the best dose for you.
What should I avoid while using Myobloc?
Myobloc may cause loss of strength or general muscle weakness or vision problems within hours to weeks of receiving Myobloc. If this happens, do not drive a car, operate machinery or do other dangerous activities. See “What is the most important information I should know about Myobloc?”
What are the possible side effects of Myobloc?
Myobloc can cause serious side effects including:
See “What is the most important information I should know about Myobloc?”
- allergic reactions. Symptoms of an allergic reaction to Myobloc may include: itching, rash, redness, swelling, wheezing, trouble breathing, or dizziness or feeling faint. Tell your doctor or get medical help right away if you get wheezing, or trouble breathing, or if you get dizzy or faint.
The most common side effects of Myobloc in people with cervical dystonia include:
- dry mouth
- injection site discomfort or pain
- trouble swallowing (dysphagia)
- headache
The most common side effects of Myobloc in people with sialorrhea include dry mouth and trouble swallowing (dysphagia).
These are not all the possible side effects of Myobloc. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to US WorldMeds at 1-888-461-2255.
General information about the safe and effective use of Myobloc
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
You can ask your doctor or pharmacist for information about Myobloc that is written for healthcare professionals.
What are the ingredients in Myobloc?
Active ingredient: botulinum toxin type B
Inactive ingredients: albumin human, sodium chloride and sodium succinate.
Label
PRINCIPAL DISPLAY PANEL – 0.5 ML VIAL CARTON
- NDC 10454-710-10
- Rx Only
- rimabotulinumtoxinB
MYOBLOC ®
Injection - FOR INTRAMUSCULAR AND
INTRAGLANDULAR USE - 2,500 Units/0.5 mL
- WARNING: Dosing units of botulinum toxins are
not interchangeable between commercial products. - Single-dose Vial
- Discard Unused Portion
- Dispense the accompanying Medication Guide to each patient.
- SOLSTICE ®
NEUROSCIENCES - US WorldMeds ®

PRINCIPAL DISPLAY PANEL – 1 ML VIAL CARTON
- NDC 10454-711-10
- Rx Only
- rimabotulinumtoxinB
MYOBLOC ®
Injection - FOR INTRAMUSCULAR AND
INTRAGLANDULAR USE - 5,000 Units/1 mL
- WARNING: Dosing units of botulinum toxins are
not interchangeable between commercial products. - Single-dose Vial
- Discard Unused Portion
- Dispense the accompanying Medication Guide to each patient.
- SOLSTICE ®
NEUROSCIENCES - US WorldMeds ®

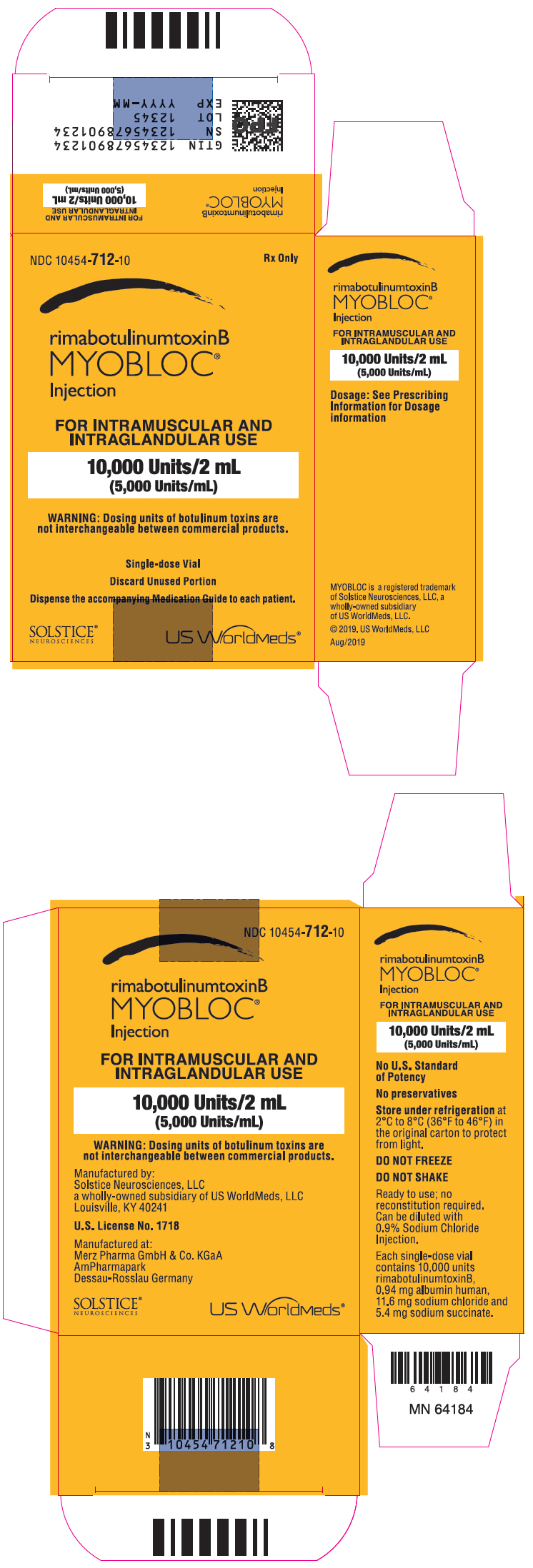
PRINCIPAL DISPLAY PANEL – 2 ML VIAL CARTON
- NDC 10454-712-10
- Rx Only
- rimabotulinumtoxinB
MYOBLOC ®
Injection - FOR INTRAMUSCULAR AND
INTRAGLANDULAR USE - 10,000 Units/2 mL
(5,000 Units/mL) - WARNING: Dosing units of botulinum toxins are
not interchangeable between commercial products. - Single-dose Vial
- Discard Unused Portion
- Dispense the accompanying Medication Guide to each patient.
- SOLSTICE ®
NEUROSCIENCES - US WorldMeds ®
SRC: NLM .
