Minolira
Generic name: minocycline (oral route)
Drug class: Tetracyclines
Medically reviewed by A Ras MD.
What is Minolira?
Minolira is prescription medicine used to treat only pimples and red bumps (non-nodular inflammatory lesions) that happen with moderate to severe acne vulgaris in people 12 years and older.
Minolira should not be used for the treatment of infections.
It is not known if Minolira is safe and effective in children under the age of 12 years.
Description
Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S (4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono hydrochloride. The structural formula is representated below:
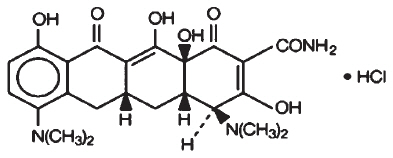
C23H27N3O7∙HCl
M. W. 493.95
MINOLIRA (minocycline hydrochloride) extended-release tablets for oral administration contain 105 mg or 135 mg of minocycline, equivalent to 113.4 mg or 145.8 mg of minocycline hydrochloride, respectively. MINOLIRA extended-release tablets, 105 mg and 135 mg, contain 25% of minocycline as immediate release beads and 75% of minocycline as extended release beads.
In addition, 105 mg and 135 mg tablets contain the following inactive ingredients: ethyl cellulose NF, hypromellose USP, isopropyl alcohol USP, microcrystalline cellulose NF, polytheylene glycol 400 NF, purified water USP, silicified microcrystalline cellulose NF, sodium stearyl fumarate NF, talc USP, triethyl citrate NF.
Both 105 mg and 135 mg tablets also contain Opadry clear which contains hydroxyl propyl cellulose NF and hypromellose USP.
Mechanism of Action
The mechanism of action of MINOLIRA for the treatment of acne is unknown.
Who should not take Minolira?
- Do not take Minolira if you are allergic to medicines in the tetracycline class. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
What should I tell my healthcare provider before taking Minolira?
Before you take Minolira, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- have liver problems
- have diarrhea or watery stools
- have vision problems.
- are pregnant or plan to become pregnant. Minolira may harm your unborn baby. Taking MINOLIRA while you are pregnant may cause serious side effects on the growth of bone and teeth of your baby. Stop taking Minolira and call your healthcare provider right away if you become pregnant during treatment with Minolira.
- are breastfeeding or plan to breastfeed. Minolira can pass into your breast milk and may harm your baby. You should not breastfeed during treatment with Minolira.
Tell your healthcare provider about all the other medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Minolira and other medicines may affect each other causing serious side effects.
Especially tell your healthcare provider if you take:
- birth control pills. Minolira may reduce the effectiveness of certain birth control pills. You could become pregnant. You should use a second form of birth control during treatment with Minolira. Talk to your healthcare provider about what types of birth control you can use to prevent pregnancy during treatment with Minolira.
- a medicine taken by mouth that contains isotretinoin.
How should I take Minolira?
See the detailed instructions for use that comes with Minolira for information about breaking your Minolira extended-release tablets.
- Take Minolira exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how much Minolira to take and if you will need to break your Minolira extended-release tablets based on your weight. Minolira comes in 2 strengths:
- 105 mg extended-release tablets
- 135 mg extended-release tablets
- Do not miss your dose of Minolira. Missing doses or not taking all doses of Minolira may:
- make the treatment not work as well
- increase the chance that the bacteria will become resistant to Minolira
- Take Minolira with or without food. Taking Minolira with food may lower your risk of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
- Do not crush or chew Minolira tablets.
- If you take too much Minolira, call your healthcare provider right away or go to the nearest hospital emergency room.
What should I avoid while taking Minolira?
- Avoid sunlight or artificial sunlight, such as sunlamps or tanning beds. You could get severe sunburn. Use sunscreen and wear loose-fitting clothes that cover your skin while out in sunlight. Stop taking Minolira if you get sunburn.
- You should not drive or operate dangerous machinery until you know how Minolira affects you. Minolira may cause you to feel dizzy or lightheaded, or have a spinning feeling (vertigo).
What are the possible side effects of Minolira?
Minolira may cause serious side effects, including:
- Harm to an unborn baby. See “What should I tell my healthcare provider before taking Minolira?”
- Permanent teeth discoloration. Minolira may permanently turn a baby or child’s teeth yellow-gray-brown during tooth development. You should not use Minolira during tooth development. Tooth development happens in the second and third trimesters of pregnancy, and from birth to 8 years of age.
- Slow bone growth. Minolira may slow bone growth in infants and children. Slow bone growth is reversible after stopping treatment with Minolira.
- Diarrhea. Diarrhea can happen with most antibiotics, including Minolira. This diarrhea may be caused by an infection (Clostridium difficile) in your intestines. Call your healthcare provider right away if you get watery or bloody stools.
- Liver problems. Minolira can cause liver problems that may lead to death. Stop taking Minolira and call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
- loss of appetite
- tiredness
- diarrhea
- yellowing of your skin or the whites of your eyes
- bleeding more easily than normal
- confusion
- sleepiness
- Increased pressure around the brain (intracranial hypertension). This condition may lead to vision changes and permanent vision loss. You may be more likely to get intracranial hypertension if you are a female of childbearing potential and are overweight or have a history of intracranial hypertension. Stop taking Minolira and tell your healthcare provider right away if you have blurred vision, double vision, vision loss, or headaches.
- Immune system reactions including a lupus-like syndrome, hepatitis, and inflammation of blood or lymph vessels (vasculitis). Call your healthcare provider right away if you get a fever, rash, joint pain, or body weakness.
- Serious skin or allergic reactions that may affect parts of your body such as your liver, lungs, kidneys and heart. Sometimes these can lead to death. Stop taking Minolira and go to the nearest hospital emergency room right away if you have any of the following signs or symptoms:
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
- blood in your urine
- fever, yellowing of the skin or the whites of your eyes, dark colored urine
- pain on the right side of the stomach area (abdominal pain)
- chest pain or abnormal heartbeats
- swelling in your legs, ankles, and feet
- darkening of your nails, skin, eyes, scars, teeth, and gums
- Discoloration (hyperpigmentation). Minolira can cause darkening of your skin, scars, teeth, gums, nails, and whites of your eyes.
The most common side effects of Minolira include:
- headache
- tiredness
- dizziness
- itching
Minolira may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
Your healthcare provider may tell you to decrease your dose or completely stop taking Minolira if you develop certain serious side effects during treatment with Minolira.
Your healthcare provider may do blood tests to check you for side effects during treatment with Minolira.
These are not all the side effects of Minolira. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Minolira
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Minolira for a condition for which it was not prescribed. Do not give Minolira to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Minolira that is written for health professionals.
How should I store Minolira?
- Store Minolira at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Minolira in the container that it comes in and keep the container tightly closed.
- Keep Minolira away from light and moisture.
Keep Minolira and all medicines out of the reach of children. General information about the safe and effective use of Minolira.
What are the ingredients in Minolira?
Active ingredient: minocycline hydrochloride.
Inactive ingredients: ethyl cellulose, hypromellose, isopropyl alcohol, microcrystalline cellulose, polyethylene glycol 400, purified water, silicified microcrystalline cellulose, sodium stearyl fumarate, talc, triethyl citrate
Both 105 mg and 135 mg tablets also contain Opadry clear which contains hydroxyl propyl cellulose and hypromellose.
Label
PRINCIPAL DISPLAY PANEL – 135 MG TABLET BOTTLE LABEL
- NDC 71403-102-30
Rx Only - minolira™
(minocycline hydrochloride)
extended-release tablets - 135 mg*
- *Each tablet contains 135 mg of minocycline
(33.75 mg as immediate release beads and
101.25 mg as extended release beads), equivalent
to 145.8 mg of minocycline hydrochloride. - 30 Tablets
- EPIHEALTH
Advancing Dermatology

PRINCIPAL DISPLAY PANEL – 105 MG TABLET BOTTLE LABEL
- NDC 71403-101-30
Rx Only - minolira™
(minocycline hydrochloride)
extended-release tablets - 105 mg*
- *Each tablet contains 105 mg of minocycline
(26.25 mg as immediate release beads and 78.75
mg as extended release beads), equivalent to
113.4 mg of minocycline hydrochloride. - 30 Tablets
- EPIHEALTH
Advancing Dermatology

