Locametz Dosage
Generic name: gallium ga 68 gozetotide
Dosage form: injection, powder, lyophilized, for solution
Medically reviewed by A Ras MD. Last updated on Apr 1, 2022.
INDICATIONS AND USAGE
- with suspected metastasis who are candidates for initial definitive therapy.
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
- for selection of patients with metastatic prostate cancer, for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated.
DOSAGE AND ADMINISTRATION
Radiation Safety – Drug Handling
After reconstitution and radiolabeling of Locametz, the vial contains gallium Ga 68 gozetotide injection. Handle the gallium Ga 68 gozetotide injection with appropriate safety measures to minimize radiation exposure.Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling gallium Ga 68 gozetotide injection.
Radiopharmaceuticals should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the governmental agency authorized to license the use of radionuclides.¶
Recommended Dosage
In adults, the recommended amount of radioactivity to be administered for PET is 111 MBq to 259 MBq (3 mCi to 7 mCi) by slow intravenous injection.
Patient Preparation
Advise patients to be well hydrated prior to gallium Ga 68 gozetotide injection administration and to void immediately prior to and frequently during the first hours after image acquisition to reduce radiation exposure.
Drug Preparation
Locametz allows the direct preparation of gallium Ga 68 gozetotide injection with the eluate from one of the following generators (see below for specific instructions for use with each generator):
- Eckert & Ziegler GalliaPharm germanium-68/gallium-68 (68Ge/68Ga) generator
- IRE ELiT Galli Eo germanium-68/gallium-68 (68Ge/68Ga) generator
The instructions for use provided by the germanium-68/gallium-68 generator manufacturer should also be followed.
Prepare gallium Ga 68 gozetotide injection according to the following aseptic procedure:
a. Use suitable shielding to reduce radiation exposure.
b. Wear waterproof gloves.
c. Flip the cap off the Locametz vial and swab the septum with an appropriate antiseptic, then allow the septum to dry.
d. Pierce the Locametz vial septum with a sterile needle connected to a 0.2-micron sterile air venting filter to maintain atmospheric pressure within the vial during the reconstitution process.
e. Place the Locametz vial in a lead shield container.
f. Follow the generator-specific procedures below. Schematic diagrams are provided in Figures 1 and 2.
Preparation with Eckert & Ziegler GalliaPharm Generator
1) Connect the male luer of the outlet line of the generator to a sterile elution needle (size 21G to 23G).
2) Connect the Locametz vial directly to the outlet line of the generator by pushing the elution needle through the rubber septum.
3) Elute directly from the generator into the Locametz vial.
4) Perform the elution manually or by means of a pump according to the generator instructions for use.
5) Reconstitute the lyophilized powder with 5 mL of the eluate.
6) At the end of the elution, disconnect the Locametz vial from the generator by removing the elution needle and the vent needle with the 0.2 micron sterile air venting filter from the rubber septum. Then, invert Locametz vial once and place it upright.
7) Incubate the Locametz vial upright between 20°C to 30°C (68°F to 86°F) for at least 5 minutes without agitation or stirring.
8) After 5 minutes, assay the vial containing the gallium Ga 68 gozetotide injection for total radioactivity using a dose calibrator, calculate the radioactivity concentration and record the result.
9) After radiolabeling, gallium Ga 68 gozetotide injection may be diluted with Sterile Water for Injection, USP or 0.9% Sodium Chloride Injection, USP up to a final volume of 10 mL.
10) Perform quality controls according to the recommended methods in order to check compliance with the specifications.
11) Store the Locametz vial containing the gallium Ga 68 gozetotide injection upright in a lead shield container below 30°C (86°F) until use.
12) After the addition of gallium-68 chloride to the Locametz vial, use gallium Ga 68 gozetotide injection within 4 hours.
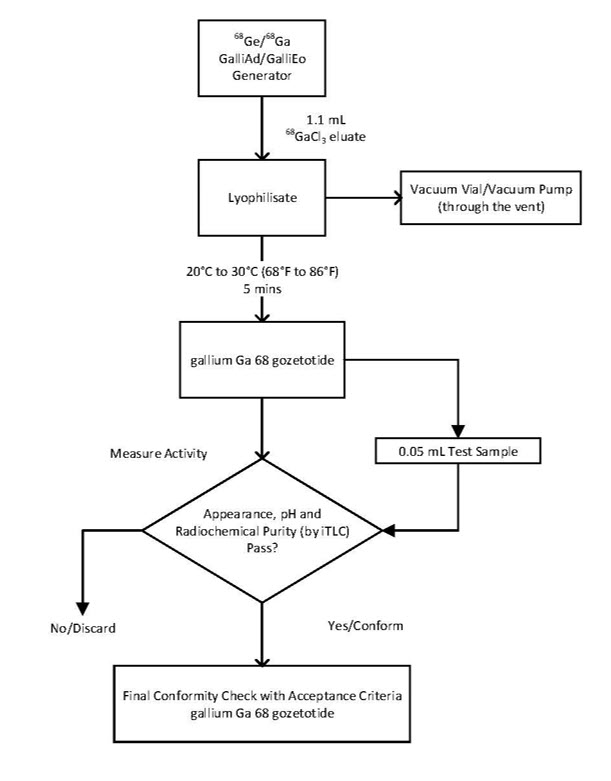
Preparation with IRE ELiT Galli Eo Generator
1) Connect the male luer of the outlet line of the generator to a sterile elution needle (size 21G to 23G).
2) Connect the Locametz vial directly to the outlet line of the generator by pushing the elution needle through the rubber septum.
3) Connect the Locametz vial through the vent needle with 0.2 micron sterile air venting filter to a vacuum vial (25 mL minimum volume) by means of a sterile needle (size 21G to 23G) or to a vacuum pump to start the elution.
4) Elute directly from the generator into the Locametz vial.
5) Reconstitute the lyophilized powder with 1.1 mL of the eluate.
6) At the end of the elution, first withdraw the sterile needle from the vacuum vial or disconnect the vacuum pump in order to establish atmospheric pressure into the Locametz vial, then disconnect the vial from the generator by removing both the elution needle and the vent needle with the 0.2 micron sterile air venting filter needle from the rubber septum. Inversion of the Locametz vial is not needed.
7) Incubate the Locametz vial upright between 20°C to 30°C (68°F to 86°F) for at least 5 minutes without agitation or stirring.
8) After 5 minutes, assay the vial containing the gallium Ga 68 gozetotide injection for total radioactivity using a dose calibrator, calculate the radioactivity concentration and record the result.
9) After radiolabeling, gallium Ga 68 gozetotide injection may be diluted with Sterile Water for Injection, USP or 0.9% Sodium Chloride Injection, USP up to a final volume of 10 mL.
10) Perform quality controls according to the recommended methods in order to check compliance with the specifications.
11) Store the Locametz vial containing the gallium Ga 68 gozetotide injection upright in a lead shield container below 30°C (86°F) until use.
12) After the addition of gallium-68 chloride to the Locametz vial, use gallium Ga 68 gozetotide injection within 4 hours.
Figure 1. Preparation with Eckert & Ziegler GalliaPharm Generator
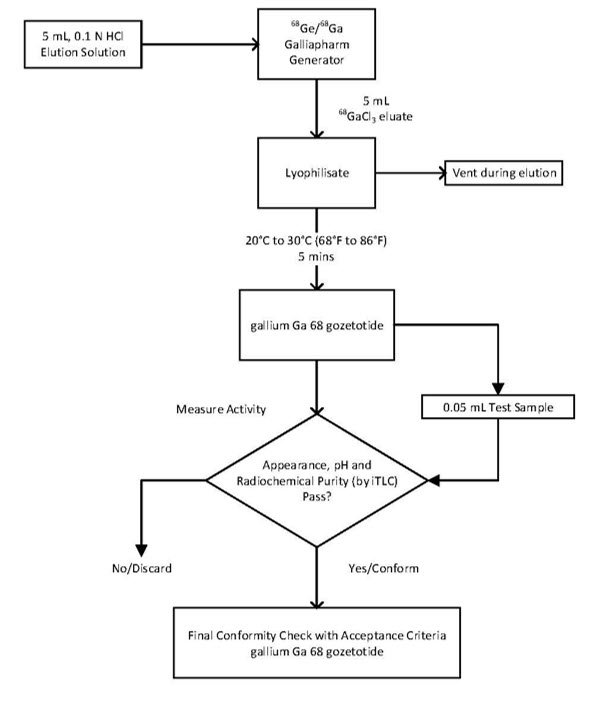
Figure 2. Preparation with IRE ELiT Galli Eo Generator
Specifications and Quality Control
Perform the quality controls in Table 1 behind a lead glass shield for radioprotection purposes.
| Test | Acceptance criteria | Method |
| Appearance | Clear, colorless, and free from particulate matter | Visual inspection |
| pH | 3.2 to 6.5 | pH-indicator strips |
| Radiochemical Purity | gallium Ga 68 gozetotide ≥ 95% Non-complexed gallium-68 species ≤ 5% |
Instant thin-layer chromatography (ITLC, see details below) |
Determine radiochemical purity of gallium Ga 68 gozetotide injection by performing instant thin-layer chromatography (ITLC).
Perform ITLC using ITLC SG strips and using ammonium acetate 1M: Methanol (1:1 V/V) as mobile phase.
ITLC Method
a. Develop the ITLC SG strip for a distance of 6 cm from the point of application (i.e., to 7 cm from the bottom of the ITLC strip).
b. Scan the ITLC SG strip with a radiometric ITLC scanner.
c. Calculate radiochemical purity by integration of the peaks on the chromatogram. Do not use the reconstituted product if the percentage (%) of non-complexed gallium-68 species is higher than 5%.
The retention factor (Rf) specifications are as follows:
- Non-complexed gallium-68 species, Rf = 0 to 0.2;
- Gallium Ga 68 gozetotide, Rf = 0.8 to 1.
Administration
a. Use aseptic technique and radiation shielding when withdrawing and administering gallium Ga 68 gozetotide injection.
b. Calculate the necessary volume to administer based on calibration time and required dose.
c. Inspect the prepared gallium Ga 68 gozetotide injection for particulate matter and discoloration behind a lead glass shield for radioprotection purposes. Use only solutions that are clear, colorless, and free from particulate matter.
d. Using a single-dose syringe fitted with a sterile needle (size 21G to 23G) and protective shielding, aseptically withdraw the prepared gallium Ga 68 gozetotide injection.
e. Verify the total radioactivity in the syringe with a dose calibrator immediately before and after gallium Ga 68 gozetotide injection administration to the patient. The dose calibrator must be calibrated with NIST traceable standards.
f. After injection of gallium Ga 68 gozetotide, administer an intravenous flush of sterile 0.9% Sodium Chloride Injection, USP to ensure full delivery of the dose.
g. Dispose of any unused gallium Ga 68 gozetotide injection in a safe manner in compliance with applicable regulations.
h. If clinically necessary, a diuretic expected to act within the uptake time period may be administered at the time of radiotracer injection to potentially decrease artifact from radiotracer accumulation in the urinary bladder and ureters.
Image Acquisition
Begin PET scanning 50 minutes to 100 minutes after the intravenous administration of gallium Ga 68 gozetotide injection. Patients should void immediately prior to image acquisition and image acquisition should begin at the mid-thighs and proceed cranially to the skull base or skull vertex. Adapt imaging techniques according to the equipment used and patient characteristics in order to obtain the best image quality possible.
Image Interpretation
Gallium Ga 68 gozetotide binds to PSMA. Based on the intensity of the signals, PET images obtained using gallium Ga 68 gozetotide injection indicate the presence of PSMA in tissues.
Imaging Prior to Initial Definitive or Suspected Recurrence Therapy
Lesions should be considered suspicious if uptake is greater than physiologic uptake in that tissue or greater than adjacent background if no physiologic uptake is expected. Tumors that do not bear PSMA will not be visualized. Increased uptake in tumors is not specific for prostate cancer.
Imaging to Select Patients for Lutetium Lu 177 Vipivotide Tetraxetan Therapy
For patient selection, interpret Locametz PET in conjunction with patients’ clinical history and imaging from other modalities, including diagnostic anatomical imaging in clinically relevant regions, such as the chest, abdomen, and pelvis. On Locametz PET images, compare uptake of gallium Ga 68 gozetotide at sites of suspected prostate cancer (lesions) with uptake in normal liver. Lesions should be considered positive if gallium Ga 68 gozetotide uptake is greater than normal liver and negative if gallium Ga 68 gozetotide uptake is less than or equal to normal liver.
Patients should be considered eligible for lutetium Lu 177 vipivotide tetraxetan therapy if at least one tumor lesion is positive and all lesions on anatomical imaging larger in short axis than size criteria are also positive [size criteria: organs ≥ 1 cm, lymph nodes ≥ 2.5 cm, bones (soft tissue component) ≥ 1 cm]. Patients should be considered ineligible for lutetium Lu 177 vipivotide tetraxetan therapy if all lesions are negative or any one lesion larger than size criteria is negative.
The interpretation of Locametz PET may differ depending on imaging readers.
For therapeutic information, refer to the prescribing information for lutetium Lu 177 vipivotide tetraxetan.
Radiation Dosimetry
Estimated radiation absorbed doses per injected activity for organs and tissues of 13 adult male patients with an early stage of disease following an intravenous bolus of gallium Ga 68 gozetotide injection are shown in Table 2.
The effective radiation dose resulting from the administration of 259 MBq (7 mCi) is 4.4 mSv. The radiation doses for this administered dose to the critical organs, which are the kidneys, urinary bladder, and spleen, are 96.2 mGy, 25.4 mGy, and 16.8 mGy, respectively.
These radiation doses are for gallium Ga 68 gozetotide injection alone. If CT or a transmission source are used for attenuation correction, the radiation dose will increase by an amount that varies by technique.
| Absorbed dose (mGy/MBq) | ||
| Organ | Mean | SD |
| Adrenals | 0.0156 | 0.0014 |
| Brain | 0.0104 | 0.0011 |
| Breasts | 0.0103 | 0.0011 |
| Gallbladder | 0.0157 | 0.0012 |
| Lower Colon | 0.0134 | 0.0009 |
| Small Intestine | 0.014 | 0.002 |
| Stomach | 0.0129 | 0.0008 |
| Heart | 0.012 | 0.0009 |
| Kidneys | 0.3714 | 0.0922 |
| Liver | 0.0409 | 0.0076 |
| Lungs | 0.0111 | 0.0007 |
| Muscle | 0.0103 | 0.0003 |
| Pancreas | 0.0147 | 0.0009 |
| Red Marrow | 0.0114 | 0.0016 |
| Skin | 0.0091 | 0.0003 |
| Spleen | 0.065 | 0.018 |
| Testes | 0.0111 | 0.0006 |
| Thymus | 0.0105 | 0.0006 |
| Thyroid | 0.0104 | 0.0006 |
| Urinary Bladder | 0.0982 | 0.0286 |
| Total Body | 0.0143 | 0.0013 |
| Effective Dose (mSv/MBq) | 0.0169 | 0.0015 |
