Isturisa
Generic name: osilodrostat
Drug class: Adrenal corticosteroid inhibitors
Medically reviewed by A Ras MD.
What is Isturisa?
Isturisa is a prescription medicine that is used to treat adults with Cushing’s diseasewho cannot have pituitary surgery, or who have had pituitary surgery, but the surgery did not cure their Cushing’s disease
It is not known if Isturisa is safe and effective in children.
Description
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor.
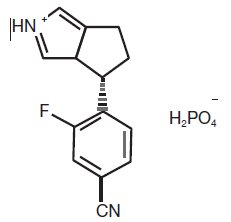
The chemical name of osilodrostat is 4-[(5R)-6,7-Dihydro-5 H-pyrrolo[1,2- c]imidazol-5-yl]-3-fluorobenzonitrile dihydrogen phosphate.
Molecular formula of osilodrostat salt (phosphate) form on anhydrous basis is: (C 13H 11FN 3) (H 2PO 4). Relative molecular mass of osilodrostat phosphate salt form is 325.24 g/mol.
ISTURISA tablets for oral administration contains 1 mg, 5 mg, or 10 mg of osilodrostat equivalent to 1.4 mg, 7.2 mg, and 14.3 mg of osilodrostat phosphate respectively, and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, and magnesium stearate. The film coat is composed of hypromellose, titanium dioxide, ferric oxide (yellow), ferric oxide (red) (1 mg and 10 mg only), ferrosoferric oxide (10 mg only), polyethylene glycol 4000, and talc.
Mechanism of Action
Osilodrostat is a cortisol synthesis inhibitor. It inhibits 11beta-hydroxylase (CYP11B1), the enzyme responsible for the final step of cortisol biosynthesis in the adrenal gland. In a Chinese hamster lung cell line V79-4 that overexpresses human CYP11B1, adrenodoxin and adrenodoxin reductase, osilodrostat inhibited the activity of human CYP11B1 dose-dependently with IC50 values of 2.5 ± 0.1 nM (n = 4).
What is the most important information I should know about Isturisa?
Isturisa can cause serious side effects, including:
- Low cortisol levels in your blood (hypocortisolism).
Tell your healthcare provider right away if you experience more than one of the following symptoms, as these may be symptoms of very low cortisol level, known as adrenal insufficiency:
- nausea
- vomiting
- tiredness (fatigue)
- low blood pressure
- stomach (abdominal) pain
- loss of appetite
- dizziness
If you get symptoms of hypocortisolism while taking Isturisa, your healthcare provider may change your dose or ask you to stop taking it.
Heart problem or a heart rhythm problem, such as an irregular heartbeat which could be a sign of a heart problem called QT prolongation. Call your healthcare provider right away if you have irregular heartbeats.
See “What are the possible side effects of Isturisa?” for more information about side effects.
What should I tell my healthcare provider before taking Isturisa?
Before you take Isturisa, tell your healthcare provider about all of your medical conditions, including if you:
- have or had heart problems, such as an irregular heartbeat, including a condition called prolonged QT syndrome (QT internal prolongation). Your healthcare provider will check the electrical signal of your heart (called an electrocardiogram) before you start taking Isturisa, 1 week after starting Isturisa, and as needed after that.
- have a history of low levels of potassium or magnesium in your blood.
- have liver problems.
- are breastfeeding or plan to breastfeed. It is not known if Isturisa passes into your breast milk.
You should not breastfeed if you take Isturisa and for 1 week after stopping treatment.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take medicines used to treat certain heart problems. Ask your healthcare provider if you are not sure whether your medicine is used to treat heart problems.
How should I take Isturisa?
- Take Isturisa exactly as your healthcare provider tells you. Your healthcare provider will tell you exactly how many tablets of Isturisa to take. Do not change your dose or stop taking Isturisa unless your healthcare provider tells you to.
- Start Isturisa by taking 2 mg two times a day by mouth or as directed by your healthcare provider.
- After you have started treatment, your healthcare provider may change your dose, depending on how you respond to the treatment with Isturisa.
- Take Isturisa with or without food.
- If you miss a dose of Isturisa, take the next dose at your regularly scheduled time.
What are the possible side effects of Isturisa?
Isturisa may cause serious side effects, including:
- See “What is the most important information I should know about Isturisa?”
- Increase in other adrenal hormone levels. Your other adrenal hormones may increase when you take Isturisa. Your healthcare provider may monitor you for the symptoms associated with these hormonal changes while you are taking Isturisa:
- Low potassium (hypokalemia).
- High blood pressure (hypertension).
- Swelling (edema) in the legs, ankles or other signs of fluid retention.
- Excessive facial or body hair growth (hirsutism).
- Acne (in women).
Call your healthcare provider if you have any of these side effects.
The most common side effects of Isturisa include:
- very low cortisol levels (adrenal insufficiency)
- tiredness (fatigue)
- nausea
- headache
- swelling of the legs, ankles or other signs of fluid retention (edema)
These are not all of the possible side effects of Isturisa.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Isturisa
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Isturisa for a condition for which it was not prescribed. Do not give Isturisa to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about Isturisa that is written for healthcare professionals.
How should I store Isturisa?
- Store Isturisa at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Isturisa dry.
Keep Isturisa and all medicines out of the reach of children.
What are the ingredients in Isturisa?
Active ingredient: osilodrostat phosphate
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, and magnesium stearate. Tablet film-coating: hypromellose, titanium dioxide, ferric oxide (yellow), ferric oxide (red) (1 mg and 10 mg only) ferrosoferric oxide (10 mg only), polyethylene glycol 4000, and talc.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

