Inbrija
Generic name: levodopa inhalation capsules
Dosage form: inhalation capsule (42 mg)
Drug class: Dopaminergic antiparkinsonism agents
Medically reviewed by A Ras MD.
What is Inbrija used for?
Inbrija is a prescription medicine that is used to treat “off” episodes (when a dose wears off) in people with Parkinson’s disease.
Description
INBRIJA consists of a dry powder formulation of levodopa for oral inhalation with the INBRIJA inhaler. The inhalation powder is packaged in white hypromellose capsules.
Each capsule contains a spray-dried powder of 42 mg levodopa active ingredient with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sodium chloride.
The active component of INBRIJA is levodopa, an aromatic amino acid. Its chemical name is (2S)-2-amino-3-(3,4-dihydroxyphenyl) propanoic acid and its structural formula is:
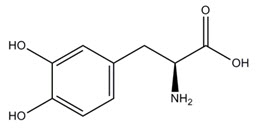
Levodopa has a molecular weight of 197.19 g/mol and molecular formula C 9H 11NO 4. Levodopa is a white to slightly off-white powder and is readily soluble in formic acid, slightly soluble in water, and practically insoluble in ethanol and diethyl ether; it dissolves in dilute hydrochloric acid.
The INBRIJA inhaler is a plastic device with a blue body, blue cap, and white mouthpiece used for inhaling INBRIJA powder.
The INBRIJA inhaler is breath-actuated by the patient. Under standardized in vitro testing conditions, the INBRIJA inhaler delivered 36.1 mg of levodopa (emitted dose) for the 42 mg capsule from the mouthpiece. No significant difference in emitted dose was observed when varying the flow rate and volume from 20 liters per minute/1L up to 90 liters per minute/2L. Peak inspiratory flow rates (PIFR) achievable through the INBRIJA inhaler were evaluated in 24 adult patients with mild to moderate Parkinson’s disease. The mean PIFR was 64 L/min (range 39–98 L/min) for patients in the ON state and 57 L/min (range 29–98 L/min) in the OFF state.
Mechanism of Action
Levodopa, the metabolic precursor of dopamine, crosses the blood-brain barrier and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson’s disease.
Before taking Inbrija, tell your doctor:
- If you are allergic to Inbrija; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have any of these health problems: Asthma or other breathing problems like COPD (chronic obstructive pulmonary disease).
- If you have taken certain drugs used for low mood (depression) like isocarboxazid, phenelzine, or tranylcypromine in the last 14 days. Taking Inbrija within 14 days of those drugs can cause very bad high blood pressure.
This is not a list of all drugs or health problems that interact with this medicine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Inbrija with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Inbrija?
- Tell all of your health care providers that you take Inbrija. This includes your doctors, nurses, pharmacists, and dentists.
- Avoid driving and doing other tasks or actions that call for you to be alert until you see how Inbrija affects you.
- To lower the chance of feeling dizzy or passing out, rise slowly if you have been sitting or lying down. Be careful going up and down stairs.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take Inbrija.
- Talk with your doctor before you drink alcohol or use other drugs and natural products that slow your actions.
- Some products may cause a dark red, brown, or black color to appear in your saliva, urine, or sweat. This is not harmful but may discolor your clothes.
- Some people have fallen asleep during activities like driving, eating, or talking. Some people did not feel sleepy and felt alert right before falling asleep. This has happened up to 1 year after Inbrija was started. If you fall asleep during activities, do not drive or do other tasks or actions that call for you to be alert while you take Inbrija. Call your doctor right away if this happens or you feel very sleepy.
- Do not stop taking Inbrija all of a sudden or lower your dose without talking to your doctor. Side effects may happen.
- If you are 65 or older, use Inbrija with care. You could have more side effects.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is Inbrija best taken?
Use Inbrija as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Use at the first signs you feel your Parkinson’s signs start to come back.
- Only use the device that comes with Inbrija. Do not use any other devices.
- Wash your hands before use.
- Be sure your hands are dry before you touch Inbrija.
- Take the capsule out of the foil right before use.
- Do not swallow capsule. The contents of the capsule will be breathed into the lungs.
- Do not open the capsules.
- Do not use capsules that are crushed, damaged, or wet.
- Put the cap back on after you are done using your dose.
- Use new inhaler with each refill.
- If you take an iron product or a multivitamin that has iron, ask your doctor or pharmacist how to take it with Inbrija. Iron may lower how well your body is able to absorb Inbrija.
What do I do if I miss a dose?
- This medicine is used on an as needed basis. Do not use more often than told by the doctor.
What are the side effects of Inbrija that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Trouble breathing that is new or worse.
- Change in the way you act.
- Hallucinations (seeing or hearing things that are not there).
- Feeling confused.
- Feeling agitated.
- Restlessness.
- Trouble sleeping.
- Strange or odd dreams.
- Strong urges that are hard to control (such as eating, gambling, sex, or spending money).
- Trouble controlling body movements that is new or worse.
- Dizziness or passing out.
- Very upset stomach or throwing up.
- Sweating a lot.
- Change in eyesight.
- Eye pain.
What are some other side effects of Inbrija?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Cough.
- Change in color of sputum.
- Signs of a common cold.
- Upset stomach.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Inbrija?
- Store at room temperature in a dry place. Do not store in a bathroom.
- Store capsules in the original container. Use right after opening.
- Do not store capsules in the inhaler.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
PRINCIPAL DISPLAY PANEL – 42 MG CAPSULE BLISTER PACK CARTON
- NDC 10144-342-60Rx ONLY
- Inbrija® (levodopa inhalation powder)
- For Oral Inhalation Only 42 mg capsules

SRC: NLM .

