Impavido
Generic name: miltefosine
Drug class: Anthelmintics
What is Impavido?
Impavido is a prescription medicine used to treat certain types of leishmaniasis visceral leishmaniasis (affecting your internal organs), cutaneous leishmaniasis (affecting the skin), mucosal leishmaniasis (affecting the nose, mouth, and throat).
It is not known if Impavido is safe and effective in children under 12 years of age.
Description
IMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent. The chemical name of miltefosine is 2-[[(hexadecyloxy)hydroxyphosphenyl]oxy]-N,N,N-trimethylethylammonium inner salt. Miltefosine is a white powder that is freely soluble in water, 0.1 N HCl or NaOH, methanol, and ethanol. It has the empirical formula of C21H46NO4P with a molecular weight of 407.6 and the following structural formula:
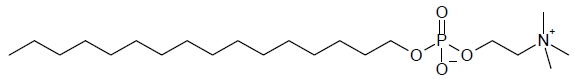
The inactive ingredients are colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
What is the most important information I should know about Impavido?
Impavido may cause serious risks to pregnancy:
- Do not take Impavido if you are pregnant. If you take Impavido during pregnancy, your baby is at risk for death or serious birth defects.
- Women who can become pregnant should use effective birth control (contraception) during Impavido treatment and for 5 months after stopping Impavido treatment. Discuss with your healthcare provider which birth control method is right for you.
- If you become pregnant while taking Impavido, tell your healthcare provider right away. Talk to your healthcare provider about taking part in the Impavido Pregnancy Registry. This is a study to learn how Impavido affects pregnancy and babies. You can enroll in this registry by calling 1-866-588-5405.
Who should not take Impavido?
Do not take Impavido if you:
- are pregnant
- have Sjögren-Larsson-Syndrome
- are allergic to miltefosine or any of the ingredients in Impavido. See the end of this leaflet for a list of the ingredients in Impavido.
- are a woman who can become pregnant and have not had a pregnancy test. Women who can get pregnant must have a urine or blood pregnancy test before taking Impavido.
- are a woman who can become pregnant and you are not willing to use effective birth control during Impavido treatment and for 5 months after treatment
What should I tell my healthcare provider before taking Impavido?
Before you take Impavido, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney or liver problems. Your healthcare provider should do blood tests to check your kidneys and liver before you start, during and after your treatment with Impavido.
- are pregnant or planning to become pregnant. Impavido may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Impavido passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Impavido. You should avoid breastfeeding while you take Impavido and for 5 months after you stop taking Impavido.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Impavido?
- Take Impavido exactly as your healthcare provider tells you to.
- Complete your full 28 day Impavido treatment.
- Take Impavido capsules whole. Do not break, crush, dissolve, or chew Impavido before swallowing.
- Take Impavido with food to help reduce stomach problems.
What are the possible side effects of Impavido?
Impavido may cause serious side effects, including:
See “What is the most important information I should know about Impavido?”
- Fertility problems in male and female rats and abnormal menstrual cycle in female dogs. It is not known if Impavido causes fertility problems in men or women
- Testicular pain and absent or decreased ejaculation
- Kidney and liver problems
- Stomach problems. Impavido can cause vomiting, diarrhea, and dehydration. Call your healthcare provider right away if you have severe vomiting and diarrhea that does not go away. Drink a lot of water to help prevent dehydration while you are having vomiting and diarrhea.
- Decreased effectiveness of oral contraceptive pills. Vomiting and diarrhea may cause your birth control pills to be less effective at preventing pregnancy. Use an extra method of birth control, such as male condoms with spermicide, until you are no longer having vomiting and diarrhea.
- Decrease in platelets (which are blood cells that help blood clot).
- Serious Skin Problems. Impavido can cause a serious skin problem called Stevens-Johnson Syndrome. If you develop a skin rash with blisters while taking Impavido, stop taking Impavido right away and call your healthcare provider.
The most common side effects of Impavido include: nausea, vomiting and diarrhea. Other side effects include abdominal pain, decreased appetite, dizziness, headache, sleepiness, skin itching, and abnormalities in liver or kidney tests.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Impavido. For more information, ask your healthcare provider.
You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Impavido
Medicines are sometimes prescribed for purposes other than those listed in this Medication Guide. Do not use Impavido for a condition for which it was not prescribed. This Medication Guide summarizes the most important information about Impavido. If you would like more information, talk to your healthcare provider or pharmacist. You can ask your healthcare or pharmacist for information about Impavido that is written for health professionals. Do not give Impavido to other people, even if they have the same symptoms that you have. For more information, go to www.IMPAVIDO.com or www.dailymed.nlm.nih.gov, or call 1-866-588-5405.
How should I store Impavido?
- Store Impavido at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect Impavido from moisture.
Keep Impavido and all medicines out of the reach of children.
What are the ingredients in Impavido?
Active ingredient: miltefosine
Inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
Label
PRINCIPAL DISPLAY PANEL – CARTON
- NDC 69051-300-01 28 Capsules
- Impavido®
(miltefosine) capsules
50 mg per Capsule - Rx only PROFOUNDA®
- Contains 2 blister cards, 14 blisters per card, 1 capsule per blister.
- Active ingredient: miltefosine
- Inactive ingredients: colloidal silicon dioxide, lactose monohydrate,
magnesium stearate, microcrystalline cellulose, talc. The capsule shell
contains gelatin, titanium dioxide, ferric oxide and purified water. - Usual dosage: see enclosed package insert for complete
prescribing information. - Keep out of reach of children.
- Store at 20-25 °C (68-77 °F); excursions permitted
to 15-30 °C (59-86 °F). [See USP Controlled Room Temperature].

SRC: NLM .
