Gilotrif
Generic name: afatinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Gilotrif used for?
Gilotrif is a prescription medicine that is used to treat lung cancer.
Description
GILOTRIF tablets contain afatinib, a tyrosine kinase inhibitor which is a 4-anilinoquinazoline. Afatinib is presented as the dimaleate salt, with the chemical name 2-butenamide, N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-,(2E)-, (2Z)-2-butenedioate (1:2). Its structural formula is:
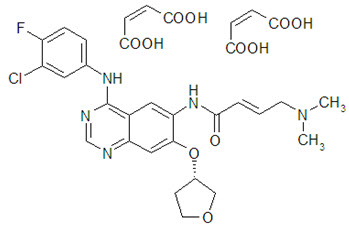
Afatinib dimaleate is a white to brownish yellow powder, water soluble and hygroscopic, with an empirical formula of C32H33ClFN5O11, and a molecular weight of 718.1 g/mol.
GILOTRIF tablets for oral administration are available in 40 mg, 30 mg, or 20 mg of afatinib (equivalent to 59.12 mg, 44.34 mg, or 29.56 mg afatinib dimaleate, respectively). The inactive ingredients of GILOTRIF are the following: Tablet Core: lactose monohydrate, microcrystalline cellulose, crospovidone, colloidal silicon dioxide, magnesium stearate and Coating: hypromellose, polyethylene glycol, titanium dioxide, talc, polysorbate 80, FD&C Blue No. 2 (40 mg and 30 mg tablets only).
Mechanism of Action
Afatinib covalently binds to the kinase domains of EGFR (ErbB1), HER2 (ErbB2), and HER4 (ErbB4) and irreversibly inhibits tyrosine kinase autophosphorylation, resulting in downregulation of ErbB signaling. Certain mutations in EGFR, including non-resistant mutations in its kinase domain, can result in increased autophosphorylation of the receptor, leading to receptor activation, sometimes in the absence of ligand binding, and can support cell proliferation in NSCLC. Non-resistant mutations are defined as those occurring in exons constituting the kinase domain of EGFR that lead to increased receptor activation and where efficacy is predicted by 1) clinically meaningful tumor shrinkage with the recommended dose of afatinib and/or 2) inhibition of cellular proliferation or EGFR tyrosine kinase phosphorylation at concentrations of afatinib sustainable at the recommended dosage according to validated methods. The most commonly found of these mutations are exon 21 L858R substitutions and exon 19 deletions.
Afatinib demonstrated inhibition of autophosphorylation and/or in vitro proliferation of cell lines expressing wild-type EGFR and in those expressing selected EGFR exon 19 deletion mutations, exon 21 L858R mutations, or other less common non-resistant mutations, at afatinib concentrations achieved in patients. In addition, afatinib inhibited in vitro proliferation of cell lines overexpressing HER2.
Treatment with afatinib resulted in inhibition of tumor growth in nude mice implanted with tumors either overexpressing wild type EGFR or HER2 or in an EGFR L858R/T790M double mutant model.
Before taking Gilotrif, tell your doctor:
- If you are allergic to Gilotrif; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you are breast-feeding. Do not breast-feed while you take Gilotrif and for 2 weeks after your last dose.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Gilotrif with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Gilotrif?
- Tell all of your health care providers that you take Gilotrif. This includes your doctors, nurses, pharmacists, and dentists.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- It is common to have diarrhea when taking Gilotrif. Some cases of diarrhea may cause fluid loss and kidney problems that can sometimes be deadly. Call your doctor right away if you have diarrhea that does not go away or if you have severe diarrhea. Do not try to treat diarrhea without first checking with your doctor.
- You may get sunburned more easily. Avoid sun, sunlamps, and tanning beds. Use sunscreen and wear clothing and eyewear that protects you from the sun.
- Very bad and sometimes deadly skin rashes have happened with Gilotrif. Talk to your doctor about any skin changes you may have.
- Very bad and sometimes deadly holes in the GI (gastrointestinal) tract have happened with Gilotrif. Talk with the doctor.
- This medicine may affect fertility. Fertility problems may lead to not being able to get pregnant or father a child.
- This medicine may cause harm to the unborn baby if you take it while you are pregnant.
- Women must use birth control while taking Gilotrif and for some time after the last dose. Ask your doctor how long to use birth control. If you get pregnant, call your doctor right away.
How is Gilotrif best taken?
Use Gilotrif as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take Gilotrif on an empty stomach. Take at least 1 hour before or at least 2 hours after a meal.
- Avoid wearing contacts unless told to wear them by your doctor.
- Keep taking Gilotrif as you have been told by your doctor or other health care provider, even if you feel well.
- If you get diarrhea, you will need to make sure to avoid getting dehydrated. Drink plenty of fluids and watch for weight loss. Talk with your doctor.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is less than 12 hours until the next dose, skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of Gilotrif that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of kidney problems like unable to pass urine, change in how much urine is passed, blood in the urine, or a big weight gain.
- Shortness of breath, a big weight gain, or swelling in the arms or legs.
- Feeling tired or weak.
- A heartbeat that does not feel normal.
- Change in eyesight, eye pain, or very bad eye irritation.
- If bright lights bother your eyes.
- Pain when passing urine.
- Redness or irritation of the palms of hands or soles of feet.
- Very bad belly pain.
- Very bad and sometimes deadly liver problems have happened with Gilotrif. Call your doctor right away if you have signs of liver problems like dark urine, feeling tired, not hungry, upset stomach or stomach pain, light-colored stools, throwing up, or yellow skin or eyes.
- Very bad and sometimes deadly lung problems have happened with Gilotrif. The chance may be raised in people who are of Asian descent. Call your doctor right away if you have new or worsening lung or breathing problems like trouble breathing, shortness of breath, cough, or fever.
- A very bad skin reaction (Stevens-Johnson syndrome/toxic epidermal necrolysis) may happen. It can cause very bad health problems that may not go away, and sometimes death. Get medical help right away if you have signs like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in your mouth, throat, nose, or eyes.
What are some other side effects of Gilotrif?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Itching.
- Dry skin.
- Dry lips.
- Pimples (acne).
- Mouth irritation or mouth sores.
- Nosebleed.
- Runny nose.
- Weight loss.
- Change in skin or finger nails.
- Diarrhea, throwing up, upset stomach, and feeling less hungry are common with Gilotrif. If these happen, talk with your doctor about ways to lower these side effects. Call your doctor right away if any of these effects bother you, do not get better, or get very bad.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Gilotrif?
- Store at room temperature in a dry place. Do not store in a bathroom.
- Store in the original container to protect from light.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
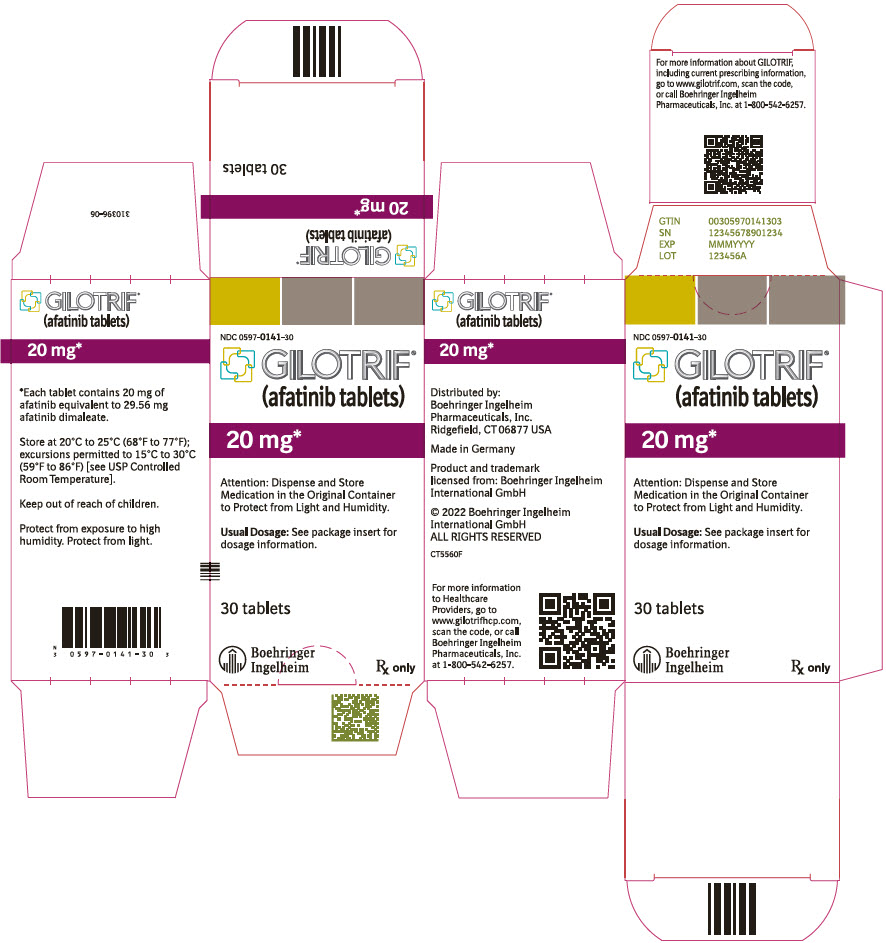
PRINCIPAL DISPLAY PANEL – 20 MG BOTTLE CARTON
- NDC 0597-0141-30
- GILOTRIF®
(afatinib tablets) - 20 mg*
- Attention: Dispense and Store
Medication in the Original Container
to Protect from Light and Humidity. - Usual Dosage: See package insert for
dosage information. - 30 tablets
- Boehringer
Ingelheim - Rx only

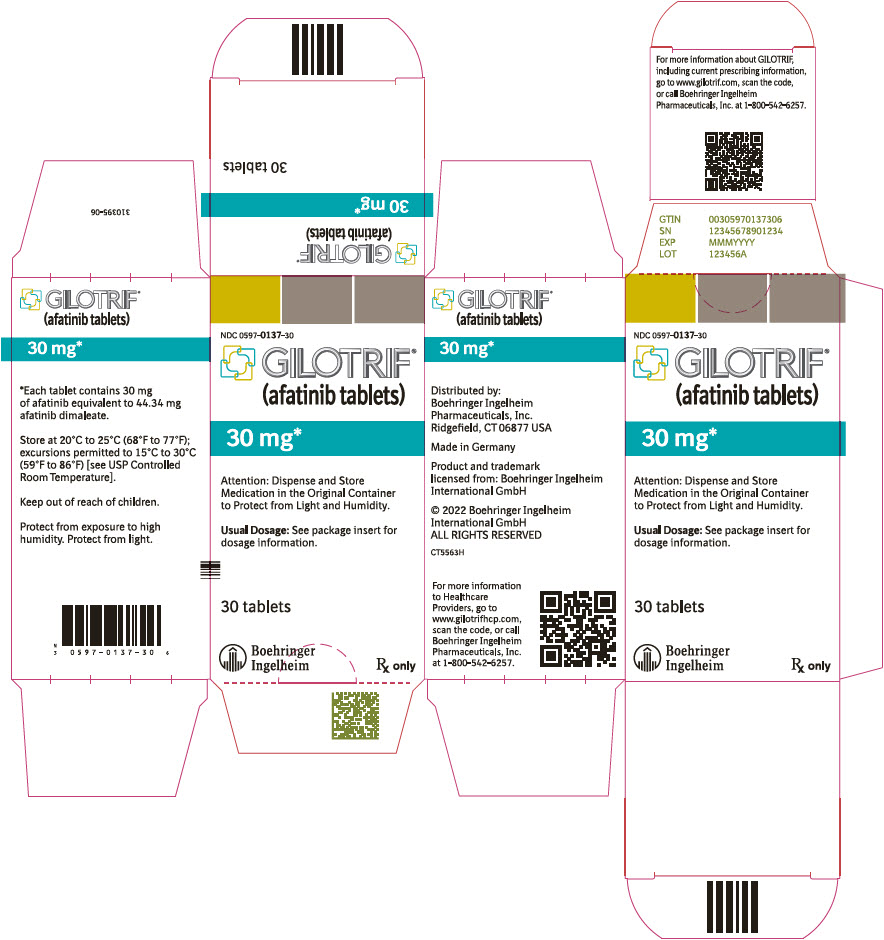
PRINCIPAL DISPLAY PANEL – 30 MG BOTTLE CARTON
- NDC 0597-0137-30
- GILOTRIF®
(afatinib tablets) - 30 mg*
- Attention: Dispense and Store
Medication in the Original Container
to Protect from Light and Humidity. - Usual Dosage: See package insert for
dosage information. - 30 tablets
- Boehringer
Ingelheim - Rx only
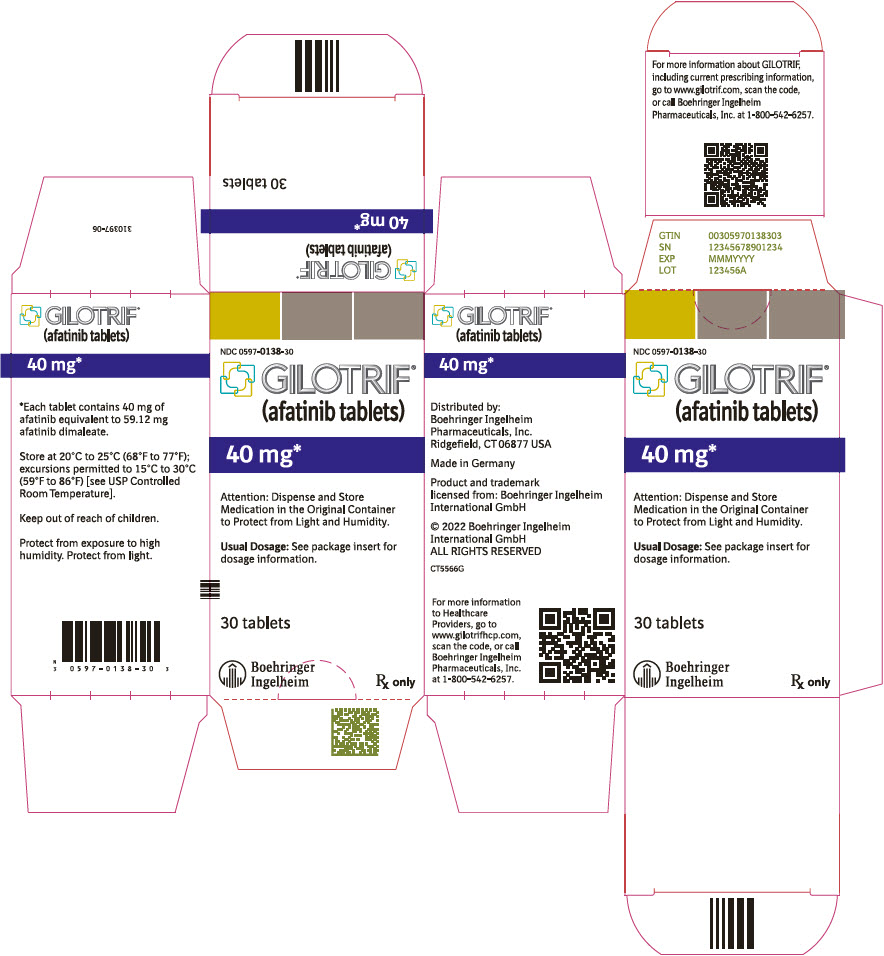
PRINCIPAL DISPLAY PANEL – 40 MG BOTTLE CARTON
- NDC 0597-0138-30
- GILOTRIF®
(afatinib tablets) - 40 mg*
- Attention: Dispense and Store
Medication in the Original Container
to Protect from Light and Humidity. - Usual Dosage: See package insert for
dosage information. - 30 tablets
- Boehringer
Ingelheim - Rx only
SRC: NLM .
