Filgrastim-aafi
Generic name: Filgrastim-aafi
Brand name: Nivestym
Drug class: Colony stimulating factors
Medically reviewed by A Ras MD.
What is filgrastim-aafi used for?
Filgrastim-aafi is a prescription medicine that is used to lower the chance of getting an infection in people with bone marrow problems caused by chemo. It is used to raise the number of white blood cells in certain patients. Filgrastim-aafi may be given to you for other reasons.
Description
Filgrastim-aafi is a 175 amino acid human granulocyte colony-stimulating factor (G-CSF) manufactured by recombinant DNA technology.
NIVESTYM is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NIVESTYM has a molecular weight of 18‚799 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NIVESTYM is produced in E coli‚ the product is non-glycosylated and thus differs from G-CSF isolated from a human cell.
NIVESTYM is a sterile‚ clear‚ colorless‚ preservative-free liquid containing filgrastim-aafi injection for subcutaneous or intravenous use. The product is available in single-dose vials and prefilled syringes. The single-dose vials contain either 300 mcg/mL or 480 mcg/1.6 mL of filgrastim-aafi. The single-dose prefilled syringes contain either 300 mcg/0.5 mL or 480 mcg/0.8 mL of filgrastim-aafi. See TABLE 4 below for product composition of each single-dose vial or prefilled syringe.
| 300mcg/mL Vial | 480 mcg/1.6 mL Vial | 300 mcg/0.5 mL Syringe | 480 mcg/0.8 mL Syringe | |
|---|---|---|---|---|
|
||||
| Filgrastim-aafi | 300 mcg | 480 mcg | 300 mcg | 480 mcg |
| Acetate | 0.59 mg | 0.94 mg | 0.295 mg | 0.472 mg |
| Polysorbate 80 | 0.04 mg | 0.064 mg | 0.02 mg | 0.032 mg |
| Sodium | 0.035 mg | 0.056 mg | 0.0175 mg | 0.028 mg |
| Sorbitol | 50 mg | 80 mg | 25 mg | 40 mg |
| Water for Injection USP q.s. ad* |
1 mL | 1.6 mL | 0.5 mL | 0.8 mL |
Mechanism of Action
Colony-stimulating factors are glycoproteins which act on hematopoietic cells by binding to specific cell surface receptors and stimulating proliferation‚ differentiation commitment‚ and some end-cell functional activation.
Endogenous G-CSF is a lineage-specific colony-stimulating factor that is produced by monocytes‚ fibroblasts, and endothelial cells. G-CSF regulates the production of neutrophils within the bone marrow and affects neutrophil progenitor proliferation‚ differentiation, and selected end-cell functions (including enhanced phagocytic ability‚ priming of the cellular metabolism associated with respiratory burst‚ antibody-dependent killing, and the increased expression of some cell surface antigens). G-CSF is not species-specific and has been shown to have minimal direct in vivo or in vitro effects on the production or activity of hematopoietic cell types other than the neutrophil lineage.
Before taking filgrastim-aafi, tell your doctor:
- If you are allergic to filgrastim-aafi; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take filgrastim-aafi with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take filgrastim-aafi?
- Tell all of your health care providers that you take filgrastim-aafi. This includes your doctors, nurses, pharmacists, and dentists.
- Do not get filgrastim-aafi at the same time or within 24 hours before or after chemo or radiation treatment. Talk with your doctor.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take filgrastim-aafi.
- This medicine may cause a severe and sometimes deadly problem called capillary leak syndrome (CLS). CLS may lead to low blood pressure, abnormal heartbeat, chest pain, or heart attack. It may also lead to lung or breathing problems, bleeding or lower blood flow in the stomach or bowel, kidney problems, swelling, or feeling confused. If you have questions, talk with the doctor.
- Some people with sickle cell disease have had times where the sickle cell disease has gotten worse when taking filgrastim-aafi. Sometimes, this has been deadly. Talk with the doctor.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is filgrastim-aafi best taken?
Use filgrastim-aafi as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into the fatty part of the skin.
- It may be given into a vein by a doctor or other healthcare provider.
- Keep taking filgrastim-aafi as you have been told by your doctor or other health care provider, even if you feel well.
- If you will be giving yourself the shot, your doctor or nurse will teach you how to give the shot.
- Before using filgrastim-aafi, take it out of the refrigerator and leave it at room temperature for 30 minutes.
- Do not use if the solution is cloudy, leaking, or has particles.
- Do not use if solution changes color.
- Do not shake the solution.
- Wash your hands before and after you give the shot.
- Do not give into skin that is irritated, tender, bruised, red, scaly, hard, scarred, or has stretch marks.
- Move the site where you give the shot with each shot.
- Throw away any part left over after the dose is given.
- Throw syringe away after use. Do not use the same syringe more than one time.
- Throw away needles in a needle/sharp disposal box. Do not reuse needles or other items. When the box is full, follow all local rules for getting rid of it. Talk with a doctor or pharmacist if you have any questions.
- If you get filgrastim-aafi on the skin, wash it off right away with soap and water.
- If you get filgrastim-aafi in the eyes, flush right away with cool water and get medical help.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of filgrastim-aafi that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of kidney problems like unable to pass urine, change in how much urine is passed, blood in the urine, or a big weight gain.
- Signs of lung or breathing problems like shortness of breath or other trouble breathing, cough, or fever.
- Dark urine.
- Chest pain or pressure or a fast heartbeat.
- Dizziness or passing out.
- Sweating a lot.
- Fast breathing.
- Coughing up blood.
- Any unexplained bruising or bleeding.
- Purple spots or redness of the skin.
- Swelling.
- Numbness or tingling.
- Enlarged and ruptured spleens have happened with filgrastim-aafi. Sometimes, ruptured spleens have been deadly. Call your doctor right away if you have left upper stomach pain or left shoulder pain.
- Swelling of the main blood vessel that comes out of the heart (aorta) has happened with filgrastim-aafi. Call your doctor right away if you feel very tired or weak. Call your doctor if you have fever, stomach pain, or back pain.
What are some other side effects of filgrastim-aafi?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Bone, joint, or muscle pain.
- Headache.
- Upset stomach or throwing up.
- Feeling tired or weak.
- Diarrhea.
- Hair loss.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out filgrastim-aafi?
- Store in a refrigerator. Do not freeze.
- Store in the original container to protect from light.
- If filgrastim-aafi freezes, let it thaw in the refrigerator before use.
- Do not use if filgrastim-aafi has been frozen more than once.
- Throw away unopened drug if left at room temperature for more than 24 hours.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
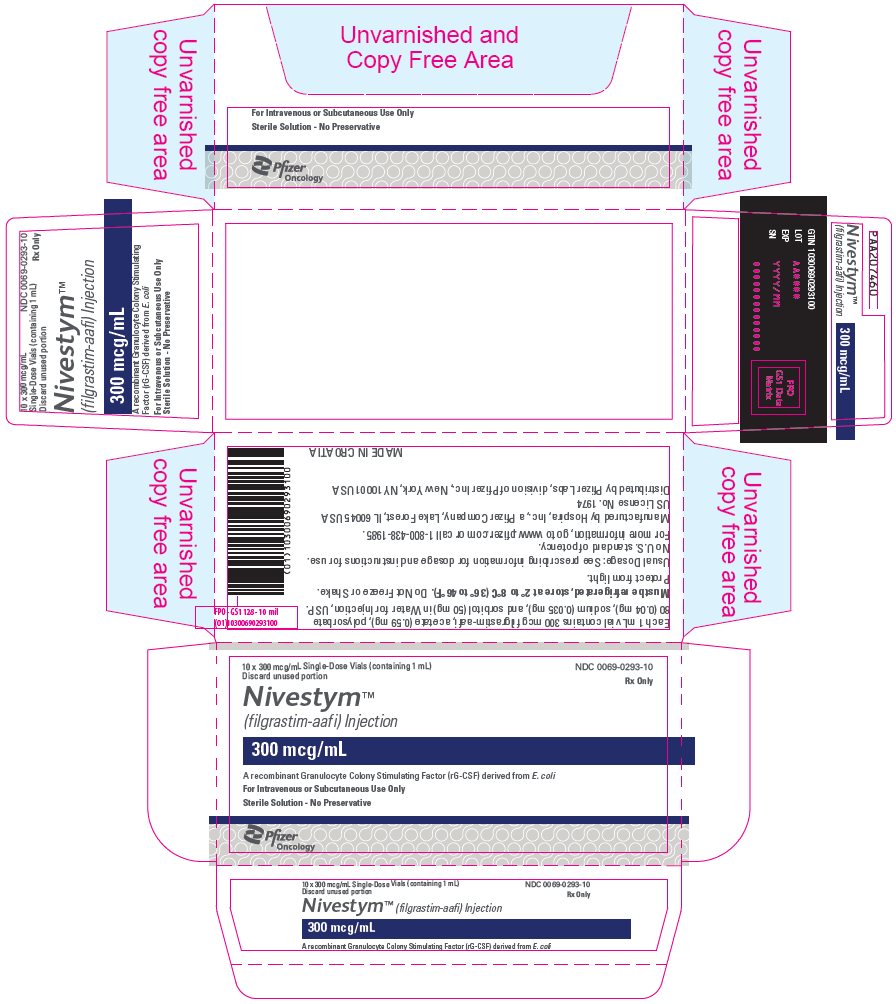
PRINCIPAL DISPLAY PANEL – 1 ML VIAL CARTON
- 10 x 300 mcg/mL Single-Dose Vials (containing 1 mL)
Discard unused portion - NDC 0069-0293-10
Rx Only - Nivestym™
(filgrastim-aafi) Injection - 300 mcg/mL
- A recombinant Granulocyte Colony Stimulating Factor (rG-CSF) derived from E. coli
- For Intravenous or Subcutaneous Use Only
- Sterile Solution – No Preservative
- Pfizer Biosimilars
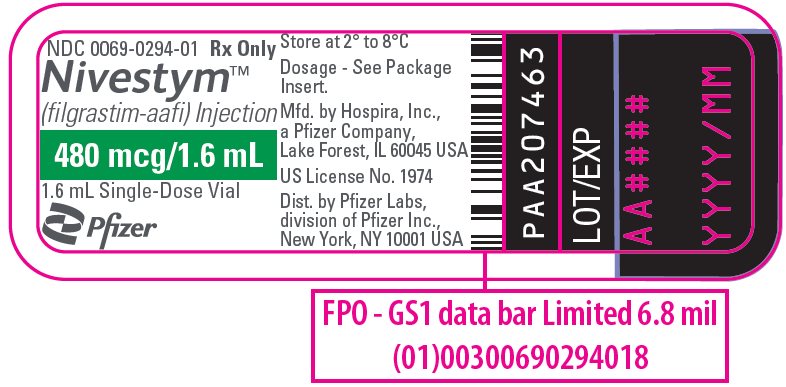
PRINCIPAL DISPLAY PANEL – 1.6 ML VIAL LABEL
- NDC 0069-0294-01
Rx Only - Nivestym™
(filgrastim-aafi) Injection - 480 mcg/1.6 mL
- 1.6 mL Single-Dose Vial
- Pfizer Biosimilars
SRC: NLM .
