Fasenra
Generic name: benralizumab
Drug class: Interleukin inhibitors
Medically reviewed by A Ras MD.
What is Fasenra?
Fasenra is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people 12 years and older whose asthma is not controlled with their current asthma medicines. Fasenra helps prevent severe asthma attacks (exacerbations) and may improve your breathing. Medicines such as Fasenra reduce blood eosinophils. Eosinophils are a type of white blood cell that may contribute to your asthma.
Fasenra is not used to treat other problems caused by eosinophils.
Fasenra is not used to treat sudden breathing problems. Tell your healthcare provider if your asthma does not get better or if it gets worse after you start treatment with Fasenra.
It is not known if Fasenra is safe and effective in children under 12 years of age.
Description
Benralizumab is a humanized monoclonal antibody (IgG1/κ-class) selective for interleukin-5 receptor alpha subunit (IL-5Rα). Benralizumab is produced in Chinese hamster ovary cells by recombinant DNA technology. Benralizumab has a molecular weight of approximately 150 kDa.
FASENRA (benralizumab) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for subcutaneous injection. Since benralizumab is a protein, a few translucent or white to off-white particles may be present in the solution. Each single-dose prefilled syringe or single-dose autoinjector delivers 1 mL containing 30 mg benralizumab, L-histidine (1.4 mg); L-histidine hydrochloride monohydrate (2.3 mg); polysorbate 20 (0.06 mg); α,α‑trehalose dihydrate (95 mg); and Water for Injection, USP. The single-dose prefilled syringe or single-dose autoinjector contains a 1 mL glass syringe with a staked 29 gauge ½ inch stainless steel needle.
Mechanism of Action
Benralizumab is a humanized afucosylated, monoclonal antibody (IgG1, kappa) that directly binds to the alpha subunit of the human interleukin-5 receptor (IL-5Rα) with a dissociation constant of 11 pM. The IL-5 receptor is expressed on the surface of eosinophils and basophils. In an in vitro setting, the absence of fucose in the Fc domain of benralizumab facilitates binding (45.5 nM) to FcɣRIII receptors on immune effector cells, such as natural killer (NK) cells, leading to apoptosis of eosinophils and basophils through antibody-dependent cell-mediated cytotoxicity (ADCC).
Inflammation is an important component in the pathogenesis of asthma. Multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) are involved in inflammation. Benralizumab, by binding to the IL-5Rα chain, reduces eosinophils through ADCC; however, the mechanism of benralizumab action in asthma has not been definitively established.
Who should not take Fasenra?
Do not use Fasenra if you are allergic to benralizumab or any of the ingredients in Fasenra. See the end of this leaflet for a complete list of ingredients in Fasenra.
What should I tell my healthcare provider before taking Fasenra?
Before using Fasenra, tell your healthcare provider about all of your medical conditions, including if you:
- are taking oral or inhaled corticosteroid medicines. Do not stop taking your corticosteroid medicines unless instructed by your healthcare provider. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.
- have a parasitic (helminth) infection.
- are pregnant or plan to become pregnant. It is not known if Fasenra will harm your unborn baby. Tell your healthcare provider if you become pregnant during your treatment with Fasenra.
- Pregnancy Registry. There is a pregnancy registry for women who use Fasenra while pregnant. The purpose of the registry is to collect information about the health of you and your baby. You can talk to your healthcare provider about how to take part in this registry or you can get more information and register by calling 1‑877-311-8972 or go to Mothertobaby.org/Fasenra.
- are breastfeeding or plan to breastfeed. It is not known if Fasenra passes into your breast milk. You and your healthcare provider should decide if you will use Fasenra and breastfeed. Talk to your healthcare provider about the best way to feed your baby if you use Fasenra.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Do not stop taking your other asthma medicines unless your healthcare provider tells you to.
How should I take Fasenra?
- Fasenra is injected under your skin (subcutaneously) one time every 4 weeks for the first 3 doses, and then every 8 weeks.
- Fasenra comes in a single dose prefilled syringe and in a single dose autoinjector.
- A healthcare provider will inject Fasenra using the single-dose prefilled syringe.
- If your healthcare provider decides that you or a caregiver can give the injection of Fasenra, you or your caregiver should receive training on the right way to prepare and give the injection using the Fasenra PEN.
- Do not try to inject Fasenra until you have been shown the right way by your healthcare provider. See the detailed “Instructions for Use” that comes with Fasenra PEN for information on how to prepare and inject Fasenra.
- If you miss a dose of Fasenra, call your healthcare provider.
What are the possible side effects of Fasenra?
Fasenra may cause serious side effects, including:
- allergic (hypersensitivity) reactions, including anaphylaxis. Serious allergic reactions can happen after you get your Fasenra injection. Allergic reactions can sometimes happen hours or days after you get your injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction:
The most common side effects of Fasenra include headache and sore throat.
These are not all the possible side effects of Fasenra.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Fasenra
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fasenra for a condition for which it was not prescribed. Do not give Fasenra to other people, even if they have the same symptoms you have. It may harm them.
You can ask your doctor or pharmacist for information about Fasenra that is written for health providers.
How should I store Fasenra?
- Store Fasenra in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Fasenra may be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 14 days.
- Once removed from the refrigerator and brought to room temperature Fasenra must be used within 14 days or thrown away.
- Store Fasenra in the original carton until you are ready to use it to protect it from light.
- Do not freeze Fasenra. Do not use Fasenra that has been frozen.
- Do not expose Fasenra to heat.
- Do not use Fasenra past the expiration date.
- Keep Fasenra and all medicines out of the reach of children.
What are the ingredients in Fasenra?
Active ingredient: benralizumab
Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 20, α,α-trehalose dihydrate, and Water for Injection.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0310-1730-30 Rx only
|
FASENRA® (benralizumab) Injection For Subcutaneous Injection Only Store the prefilled syringe refrigerated at 36°F – 46°F (2°C – 8°C) in original carton to protect from light. Do not shake, freeze, or expose to heat. |
30 mg/mL 1 single-dose prefilled syringe Discard unused portion. AstraZeneca |

PACKAGE/LABEL DISPLAY PANEL
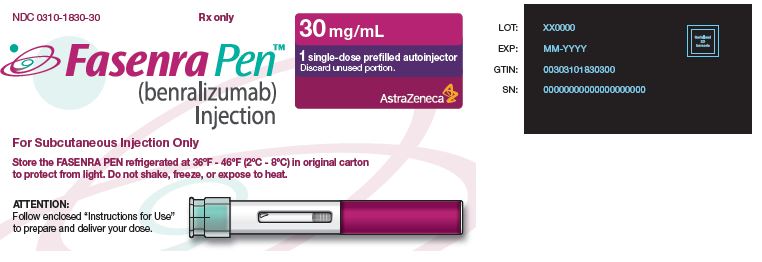
NDC 0310-1830-30 Rx only
|
FASENRA PEN™ (benralizumab) Injection For Subcutaneous Injection Only Store the FASENRA PEN refrigerated at 36°F – 46°F (2°C – 8°C) in original carton to protect from light. Do not shake, freeze, or expose to heat. |
30 mg/mL 1 single-dose prefilled autoinjector Discard unused portion. AstraZeneca |
SRC: NLM .
