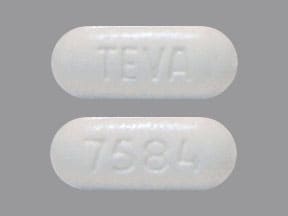
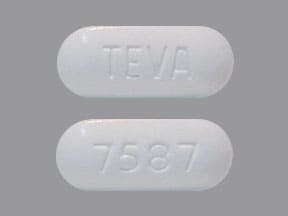
Ezetimibe and Simvastatin
Generic name: ezetimibe and simvastatin
Brand name: Vytorin
Dosage form: oral tablet (10 mg-10 mg; 10 mg-20 mg; 10 mg-40 mg; 10 mg-80 mg)
Drug class: Antihyperlipidemic combinations
Medically reviewed by A Ras MD.
What is ezetimibe and simvastatin?
Ezetimibe and Simvastatin is used to lower bad cholesterol and raise good cholesterol (HDL). It is used to lower triglycerides.
Description
Ezetimibe and Simvastatin Tablets contain ezetimibe USP, a selective inhibitor of intestinal cholesterol and related phytosterol absorption, and simvastatin USP, an HMG-CoA reductase inhibitor.
The chemical name of ezetimibe, USP is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The molecular formula is C24H21F2NO3 and its molecular weight is 409.4.
Ezetimibe, USP is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Its structural formula is:

Simvastatin, USP an inactive lactone, is hydrolyzed to the corresponding β-hydroxyacid form, which is an inhibitor of HMG-CoA reductase. Simvastatin, USP is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1α,3α,7β,8β(2S*,4S*),-8aβ]]. The molecular formula of simvastatin, USP is C25H38O5 and its molecular weight is 418.57.
Simvastatin, USP is a white to off-white, nonhygroscopic, crystalline powder that is practically insoluble in water and freely soluble in chloroform, methanol and ethanol. Its structural formula is:

Ezetimibe and simvastatin is available for oral use as tablets containing 10 mg of ezetimibe USP, and 10 mg of simvastatin, USP (ezetimibe and simvastatin tablets 10 mg/10 mg), 20 mg of simvastatin, USP (ezetimibe and simvastatin tablets 10 mg/20 mg), 40 mg of simvastatin, USP (ezetimibe and simvastatin tablets 10 mg/40 mg), or 80 mg of simvastatin, USP (ezetimibe and simvastatin tablets 10 mg/80 mg). Each tablet contains the following inactive ingredients: ascorbic acid, butylated hydroxyanisole, citric acid anhydrous, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and propyl gallate.
Mechanism of Action
Ezetimibe and Simvastatin
Plasma cholesterol is derived from intestinal absorption and endogenous synthesis. Ezetimibe and simvastatin contains ezetimibe and simvastatin, two lipid-lowering compounds with complementary mechanisms of action. Ezetimibe and simvastatin reduces elevated total-C, LDL-C, Apo B, TG, and non-HDL-C, and increases HDL-C through dual inhibition of cholesterol absorption and synthesis.
Ezetimibe
Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. The molecular target of ezetimibe has been shown to be the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is involved in the intestinal uptake of cholesterol and phytosterols. In a 2-week clinical study in 18 hypercholesterolemic patients, ezetimibe inhibited intestinal cholesterol absorption by 54%, compared with placebo. Ezetimibe had no clinically meaningful effect on the plasma concentrations of the fat-soluble vitamins A, D, and E and did not impair adrenocortical steroid hormone production.
Ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of statins.
Simvastatin
Simvastatin is a prodrug and is hydrolyzed to its active β-hydroxyacid form, simvastatin acid, after administration. Simvastatin is a specific inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in the biosynthetic pathway for cholesterol. In addition, simvastatin reduces very-low-density lipoproteins (VLDL) and TG and increases HDL-C.
Before taking ezetimibe and simvastatin, tell your doctor:
- If you are allergic to ezetimibe and simvastatin; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you take any drugs (prescription or OTC, natural products, vitamins) that must not be taken with ezetimibe and simvastatin, like certain drugs that are used for HIV, infections, or depression. There are many drugs that must not be taken with ezetimibe and simvastatin. Your doctor or pharmacist can tell you if you are taking a drug that must not be taken with ezetimibe and simvastatin.
- If you have liver disease or raised liver enzymes.
- If you are pregnant or may be pregnant. Do not take ezetimibe and simvastatin if you are pregnant.
- If you are breast-feeding. Do not breast-feed while you take ezetimibe and simvastatin.
This is not a list of all drugs or health problems that interact with ezetimibe and simvastatin.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take ezetimibe and simvastatin with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take ezetimibe and simvastatin?
- Tell all of your health care providers that you take ezetimibe and simvastatin. This includes your doctors, nurses, pharmacists, and dentists.
- If you have high blood sugar (diabetes), you will need to watch your blood sugar closely.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Follow the diet and workout plan that your doctor told you about.
- Do not take colestipol, cholestyramine, or colesevelam within 4 hours before or 2 hours after ezetimibe and simvastatin.
- Avoid or limit drinking alcohol to less than 3 drinks a day. Drinking too much alcohol may raise your chance of liver disease.
- Avoid grapefruit and grapefruit juice.
- If you are Chinese, talk with your doctor. You could have a greater risk of muscle problems.
- If you are 65 or older, use ezetimibe and simvastatin with care. You could have more side effects.
- This medicine may cause harm to the unborn baby if you take it while you are pregnant. If you are pregnant or you get pregnant while taking ezetimibe and simvastatin, call your doctor right away.
- Use birth control that you can trust to prevent pregnancy while taking ezetimibe and simvastatin.
How is ezetimibe and simvastatin best taken?
Use ezetimibe and simvastatin as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take in the evening.
- Take with or without food.
- Keep taking ezetimibe and simvastatin as you have been told by your doctor or other health care provider, even if you feel well.
What do I do if I miss a dose?
- Skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of ezetimibe and simvastatin that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Not able to pass urine or change in how much urine is passed.
- This medicine may cause muscle pain, tenderness, or weakness. Sometimes, a very bad muscle problem may happen that may lead to kidney problems. Rarely, deaths have happened in people who get these problems when taking drugs like this one. Call your doctor right away if you have muscle pain, tenderness, or weakness that is not normal (with or without fever or feeling out of sorts). Call your doctor right away if you have muscle signs that last after your doctor has told you to stop taking ezetimibe and simvastatin.
- Liver problems have happened with drugs like this one. Sometimes, this has been deadly. Call your doctor right away if you have signs of liver problems like dark urine, feeling tired, not hungry, upset stomach or stomach pain, light-colored stools, throwing up, or yellow skin or eyes.
What are some other side effects of ezetimibe and simvastatin?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Headache.
- Signs of a common cold.
- Diarrhea.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out ezetimibe and simvastatin?
- Store at room temperature.
- Keep lid tightly closed.
- Protect from light.
- Store in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
PRINCIPAL DISPLAY PANEL
- NDC 45963-565-30
Ezetimibe and Simvastatin Tablets
10 mg/10 mg - Actavis
30 Tablets
Rx Only

PRINCIPAL DISPLAY PANEL
- NDC 45963-566-30
Ezetimibe and Simvastatin Tablets
10 mg/20 mg - Actavis
30 Tablets
Rx Only

PRINCIPAL DISPLAY PANEL
- NDC 45963-567-30
Ezetimibe and Simvastatin Tablets
10 mg/40 mg - Actavis
30 Tablets
Rx Only

PRINCIPAL DISPLAY PANEL
- NDC 45963-568-30
Ezetimibe and Simvastatin Tablets
10 mg/80 mg - Actavis
30 Tablets
Rx Only

SRC: NLM .