Evista
Generic name: raloxifene
Drug classes: Hormones / antineoplastics, Selective estrogen receptor modulators
Medically reviewed by A Ras MD.
What is Evista?
Evista is a type of prescription medicine called a Selective Estrogen Receptor Modulator (SERM). Evista is for women after menopause, and has more than one use:
You and your doctor should talk about whether the possible benefit of Evista in lowering your chance of getting invasive breast cancer is greater than its possible risks.
Osteoporosis: Evista treats and prevents osteoporosis by helping make your bones stronger and less likely to break.
Invasive Breast Cancer: If you have osteoporosis or are at high risk for breast cancer, Evista can be used to lower your chance of getting invasive breast cancer. Evista will not totally get rid of your chance of getting breast cancer. Your doctor can estimate your risk of breast cancer by asking you about risk factors, including your age (getting older) family history of breast cancer in your mother, sister, or daughter, a history of any breast biopsy, especially an abnormal biopsy.
Evista is not for use in premenopausal women (women who have not passed menopause).
Description
EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:
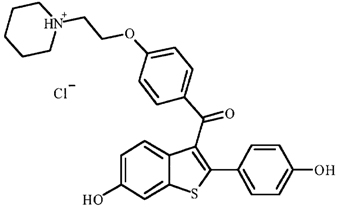
The chemical designation is methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl]-[4-[2-(1-piperidinyl)ethoxy]phenyl]-, hydrochloride. Raloxifene hydrochloride (HCl) has the empirical formula C28H27NO4S•HCl, which corresponds to a molecular weight of 510.05. Raloxifene HCl is an off-white to pale-yellow solid that is very slightly soluble in water.
EVISTA is supplied in a tablet dosage form for oral administration. Each EVISTA tablet contains 60 mg of raloxifene HCl, which is the molar equivalent of 55.71 mg of free base. Inactive ingredients include anhydrous lactose, carnauba wax, crospovidone, FD&C Blue No. 2 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, modified pharmaceutical glaze, polyethylene glycol, polysorbate 80, povidone, propylene glycol, and titanium dioxide.
Mechanism of Action
Raloxifene is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM). The biological actions of raloxifene are largely mediated through binding to estrogen receptors. This binding results in activation of estrogenic pathways in some tissues (agonism) and blockade of estrogenic pathways in others (antagonism). The agonistic or antagonistic action of raloxifene depends on the extent of recruitment of coactivators and corepressors to estrogen receptor (ER) target gene promoters.
Raloxifene appears to act as an estrogen agonist in bone. It decreases bone resorption and bone turnover, increases bone mineral density (BMD) and decreases fracture incidence. Preclinical data demonstrate that raloxifene is an estrogen antagonist in uterine and breast tissues. These results are consistent with findings in clinical trials, which suggest that EVISTA lacks estrogen-like effects on the uterus and breast tissue.
What is the most important information I should know about Evista?
Serious and life-threatening side effects can occur while taking Evista. These include blood clots and dying from stroke:
- Increased risk of blood clots in the legs (deep vein thrombosis) and lungs (pulmonary embolism) have been reported with Evista. Women who have or have had blood clots in the legs, lungs, or eyes should not take Evista.
- Women who have had a heart attack or are at risk for a heart attack may have an increased risk of dying from stroke when taking Evista.
1. Before starting Evista, tell your doctor if you have had blood clots in your legs, lungs, or eyes, a stroke, mini-stroke (transient ischemic attack), or have an irregular heartbeat.
2. Stop taking Evista and call your doctor if you have:
- leg pain or a feeling of warmth in the lower leg (calf).
- swelling of the legs, hands, or feet.
- sudden chest pain, shortness of breath, or coughing up blood.
- sudden change in your vision, such as loss of vision or blurred vision.
3. Being still for a long time (such as sitting still during a long car or airplane trip or being in bed after surgery) can increase your risk of blood clots. (See “What should I avoid if I am taking Evista?”)
Who should not take Evista?
Do not take Evista if you:
- have or have had blood clots in your legs, lungs, or eyes. Taking Evista may increase the risk of getting blood clots.
- are pregnant or could become pregnant. Evista could harm your unborn child.
- are nursing a baby. It is not known if Evista passes into breast milk or what effect it might have on the baby.
What should I tell my healthcare provider before taking Evista?
Evista may not be right for you. Before taking Evista, tell your doctor about all your medical conditions, including if you:
- have had blood clots in your legs, lungs, or eyes, a stroke, mini-stroke (TIA/transient ischemic attack), or a type of irregular heartbeat (atrial fibrillation).
- have had breast cancer. Evista has not been fully studied in women who have a history of breast cancer.
- have liver or kidney problems.
- have taken estrogen in the past and had a high increase of triglycerides (a kind of fat in the blood).
- are pregnant, planning to become pregnant, or breast-feeding (see “Who should not take Evista?”).
Tell your doctor about all medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist each time you get a new medicine. Especially tell your doctor if you take*:
- warfarin (Coumadin, Jantoven)
- If you are taking warfarin or other coumarin blood thinners, your doctor may need to do a blood test when you first start or if you need to stop taking Evista. Names for this test include “prothrombin time,” “pro-time,” or “INR.” Your doctor may need to adjust the dose of your warfarin or other coumarin blood thinner.
- cholestyramine
- estrogens
Evista should not be taken with cholestyramine or estrogens.
How should I take Evista?
- Take Evista exactly how your doctor tells you to.
- Keep taking Evista for as long as your doctor prescribes it for you. It is not known how long you should keep taking Evista to lower your chance of getting invasive breast cancers.
- It is important to get your refills on time so you do not run out of the medicine.
- Take one Evista tablet each day.
- Take Evista at any time of the day, with or without food.
- To help you remember to take Evista, it may be best to take it at about the same time each day.
- Calcium and vitamin D may be taken at the same time as Evista. It is important to take calcium and vitamin D, as directed by your physician, to prevent or treat osteoporosis.
- If you miss a dose, take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and take only your next regularly scheduled dose. Do not take two doses at the same time.
What should I avoid while taking Evista?
- Being still for a long time (such as during long trips or being in bed after surgery) can increase the risk of blood clots. Evista may add to this risk. If you will need to be still for a long time, talk with your doctor about ways to reduce the risk of blood clots. On long trips, move around periodically. Stop taking Evista at least 3 days before a planned surgery or before you plan on being still for a long time. You should start taking Evista again when you return to your normal activities.
- Some medicines should not be taken with Evista (see “What should I tell my doctor before taking Evista?”).
What are the possible side effects of Evista?
Serious and life-threatening side effects can occur while taking Evista. These include blood clots and dying from stroke:
- Increased risk of blood clots in the legs (deep vein thrombosis) and lungs (pulmonary embolism) have been reported with Evista. Women who have or have had blood clots in the legs, lungs, or eyes should not take Evista.
- Women who have had a heart attack or are at risk for a heart attack may have an increased risk of dying from stroke when taking Evista.
See “What is the most important information I should know about Evista?”
The most common side effects of Evista are hot flashes, leg cramps, swelling of the feet, ankles, and legs, flu syndrome, joint pain, and sweating. Hot flashes are more common during the first 6 months after starting treatment.
These are not all the side effects of Evista. Tell your doctor about any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What else should I know about Evista?
- Do not use Evista to prevent heart disease, heart attack, or strokes.
- To get the calcium and vitamin D you need, your doctor may advise you to change your diet and/or take supplemental calcium and vitamin D. Your doctor may suggest other ways to help treat or prevent osteoporosis, in addition to taking Evista and getting the calcium and vitamin D you need. These may include regular exercise, stopping smoking, and drinking less alcohol.
- Women who have hot flashes can take Evista. Evista does not treat hot flashes, and it may cause hot flashes in some women.
- Evista has not been found to cause breast tenderness or enlargement. If you notice any changes in your breasts, call your doctor to find out the cause. Before starting and while taking Evista you should have breast exams and mammograms, as directed by your doctor. Because Evista does not eliminate the chance of developing breast cancers, you need these examinations to find any breast cancers as early as possible.
- Evista should not cause spotting or menstrual-type bleeding. If you have any vaginal bleeding, call your doctor to find out the cause. Evista has not been found to increase the risk for cancer of the lining of the uterus.
- Women in clinical trials have taken Evista for up to eight years.
General Information about the safe and effective use of Evista
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Evista for a condition for which it was not prescribed. Do not give your Evista to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide is a summary of the most important information about Evista. If you would like more information about Evista, talk with your doctor. You can ask your doctor or pharmacist for information about Evista that is written for health professionals. For more information, call 1-800-545-5979 (toll-free).
How should I store Evista?
- Store Evista at 68°F to 77°F (20°C-25°C).
- Keep Evista and all medicines out of the reach of children.
What are the ingredients in Evista?
Active Ingredient: raloxifene hydrochloride
Inactive Ingredients: anhydrous lactose, carnauba wax, crospovidone, FD&C Blue No. 2 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, modified pharmaceutical glaze, polyethylene glycol, polysorbate 80, povidone, propylene glycol, and titanium dioxide.
Label
PACKAGE LABEL – Evista 60mg 30ct Bottle (0002-4184)
- NDC 0002-4184-30
- 30 Tablets
- No. 4165
- EVISTA®
- raloxifene HCl tablets 60mg
- 4165
- Rx only
- Lilly


