Enstilar
Generic name: betamethasone and calcipotriene topical
Brand names: Enstilar, Taclonex, Wynzora
Drug class: Topical antipsoriatics
Medically reviewed by A Ras MD.
What is Enstilar Foam?
Enstilar Foam is a prescription medicine used on the skin (topical) to treat plaque psoriasis in people 12 years of age and older. It is not known if Enstilar Foam is safe and effective in children under 12 years of age.
Description
Enstilar Foam contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only.
Calcipotriene Hydrate
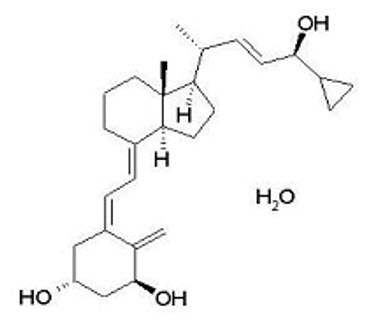
Calcipotriene hydrate is a synthetic vitamin D analog and has the chemical name 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol,24-cyclo-propyl-,monohydrate, (1α,3β,5Z,7E,22E,24S) with the empirical formula C27H40O3∙H2O), a molecular weight of 430.6, and the following structural formula (calcipotriene hydrate is a white to almost white, crystalline compound):
Betamethasone Dipropionate
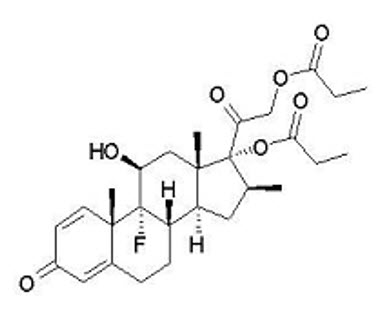
Betamethasone dipropionate is a synthetic corticosteroid and has the chemical name pregna-1,4-diene-3,20-dione-9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxypropoxy)-(11β,16β), with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula (betamethasone dipropionate is a white to almost white, crystalline powder):
Enstilar® Foam
Each gram of Enstilar Foam contains 50 mcg of calcipotriene (equivalent to 52.2 mcg of calcipotriene hydrate) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone) in a base of white petrolatum, polyoxypropylene stearyl ether, mineral oil, all-rac-alpha-tocopherol, and butylhydroxytoluene. Enstilar Foam is a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. The propellants used in Enstilar Foam are dimethyl ether and butane. At administration, the product is a white to off-white foam after evaporation of the propellants. Enstilar Foam has the appearance of a non-expanding foam that gradually collapses after spraying.
Mechanism of Action
Enstilar Foam combines the pharmacological effects of calcipotriene hydrate as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic corticosteroid. However, while their pharmacologic and clinical effects are known, the exact mechanisms of their actions in the treatment of plaque psoriasis are unknown.
What is the most important information I should know about Enstilar Foam?
Important: Enstilar Foam is for use on skin only (topical). Do not get Enstilar Foam near or in your mouth, eyes, or vagina.
There are other medicines that contain the same medicine that is in Enstilar Foam and are used to treat plaque psoriasis. Do not use other products containing calcipotriene or a corticosteroid medicine with Enstilar Foam without talking to your healthcare provider first.
What should I tell my healthcare provider before using Enstilar?
Before you use Enstilar Foam, tell your heathcare provider about all of your medical conditions, including if you:
- have a calcium metabolism disorder.
- have thinning skin (atrophy) at the treatment site.
- are pregnant or plan to become pregnant. It is not known if Enstilar Foam will harm your unborn baby. Enstilar Foam may increase your chance of having a low birth weight baby. If you use Enstilar Foam during pregnancy, use Enstilar Foam on the smallest area of the skin and for the shortest time needed.
- are breastfeeding or plan to breastfeed. It is not known if Enstilar Foam passes into your breast milk. Breastfeeding women should use Enstilar Foam on the smallest area of the skin and for the shortest time needed. Do not apply Enstilar Foam directly to your nipple and areola to avoid contact with your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I use Enstilar Foam?
See the instructions for use for detailed information about the right way to use Enstilar Foam.
- Use Enstilar Foam exactly as your healthcare provider tells you to use it.
- Your healthcare provider should tell you how much Enstilar Foam to use and where to use it.
- Apply Enstilar Foam to the affected areas of your skin 1 time a day for up to 4 weeks. You should stop treatment when your plaque psoriasis is under control unless your healthcare provider gives you other instructions.
- Do not use more than 60 grams of Enstilar Foam every 4 days.
- Do not use Enstilar Foam longer than prescribed. Using too much Enstilar Foam, or using it too often, or for too long can increase your risk for having serious side effects.
- Shake the Enstilar Foam can before you use it.
- Avoid using Enstilar Foam on your face, groin, or armpits, or if you have thinning of your skin (atrophy) at the treatment site.
- If you accidentally get Enstilar Foam on your face, in your mouth or in your eyes, wash the area with water right away.
- Wash your hands after using Enstilar Foam unless you are using the medicine to treat your hands.
- Do not bandage or cover the treated skin area, unless instructed by your healthcare provider.
What should I avoid while using Enstilar Foam?
Enstilar Foam is flammable. Avoid fire, flame, and smoking when applying and right after you apply Enstilar Foam.
What are the possible side effects of Enstilar Foam?
Enstilar Foam may cause serious side effects, including:
- Too much calcium in your blood or urine. Your healthcare provider may tell you to stop or temporarily stop treatment with Enstilar Foam if you have too much calcium in your blood or urine. Your healthcare provider may do blood and urine tests to check your calcium levels and adrenal gland function while you are using Enstilar Foam.
- Enstilar Foam can pass through your skin. Too much Enstilar Foam passing through your skin can cause your adrenal glands to stop working properly. Your healthcare provider may do blood tests to check for adrenal gland problems. Your healthcare provider may tell you to stop or temporarily stop treatment with Enstilar Foam.
- Cushing’s syndrome, a condition that happens when your body is exposed to too much of the hormone cortisol.
- High blood sugar (hyperglycemia) and sugar in your urine
- Skin problems, including reactions where Enstilar Foam is applied, and allergic reactions (allergic contact dermatitis). Tell your healthcare provider if you have any skin problems, including:
- thinning of your skin
- burning
- inflammation
- itiching
- irritation
- dryness
- changes in skin color
- redness
- infection
- raised bumps on your skin
- Eye problems. Using Enstilar Foam may increase your chance of getting cataracts and glaucoma. Do not get Enstilar Foam in your eyes because it may cause eye irritation. Tell your healthcare provider if you have blurred vision or other vision problems during treatment with Enstilar Foam.
The most common side effects of Enstilar Foam include:
- irritation
- itching
- inflamed hair pores (folliculitis)
- changes in skin color
- rash with raised red bumps or skin welts (hives)
- worsening of your psoriasis
These are not all the possible side effects of Enstilar Foam.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Enstilar Foam.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Enstilar Foam for a condition for which it was not prescribed. Do not give Enstilar Foam to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Enstilar Foam that is written for health professionals.
How should I store Enstilar Foam?
- Store Enstilar Foam at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not expose Enstilar Foam to heat or store at temperatures above 120°F (49°C).
- Do not puncture or burn the Enstilar Foam can.
- Do not freeze Enstilar Foam.
- Enstilar Foam has an expiration date (exp.) marked on the can. Do not use after this date.
- Throw away (dispose of) unused Enstilar Foam 6 months after the can has been opened.
Keep Enstilar Foam and all medicines out of the reach of children.
What are the ingredients in Enstilar Foam?
Active ingredients: calcipotriene and betamethasone dipropionate.
Inactive ingredients: white petrolatum, polyoxypropylene stearyl ether, mineral oil, all-rac-alpha-tocopherol, and butylhydroxytoluene.
Propellants: dimethyl ether and butane.
Instructions for use for Enstilar Foam
Enstilar [En-still-ar]
Calcipotriene and betamethasone dipropionate
Foam
Important Information You Need to Know Before Applying Enstilar Foam:
Enstilar Foam is for use on skin only (topical). Do not get Enstilar Foam near or in your mouth, eyes or vagina. If you accidentally get Enstilar Foam on the face, in the mouth or in the eyes, wash the area with water right away. Do not swallow Enstilar Foam.
Applying Enstilar Foam:
Follow your healthcare provider’s instructions on how much Enstilar Foam to use and where to use it.
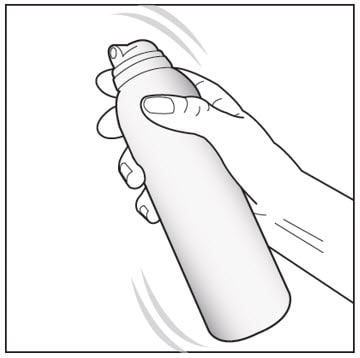
Wash your hands before applying Enstilar Foam.
Step 1: Remove the cap from the can. Shake the can before use.
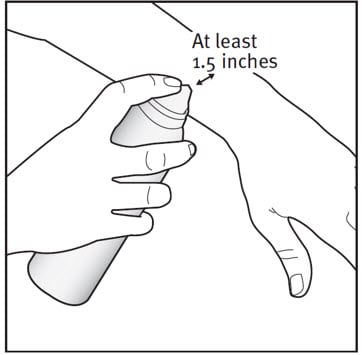
Step 2: Hold the can at least 1.5 inches from the affected area.
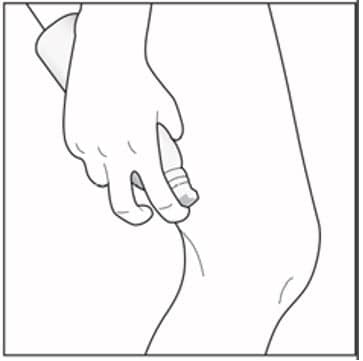
Step 3: The foam can be sprayed holding the can in any position except sideways (horizontally). To spray, push down on the nozzle. Note: Enstilar Foam will slowly become smaller in size after spraying.
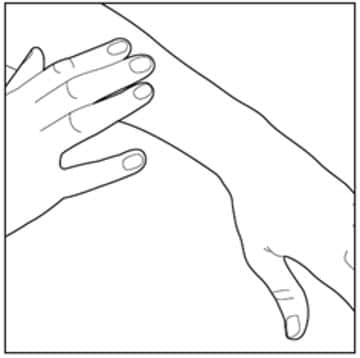
Step 4: Gently rub in Enstilar Foam into your affected skin area. Repeat the steps above to apply Enstilar Foam to other affected areas as instructed by your healthcare provider.
Step 5: After applying Enstilar Foam, put the cap back on the can.
Step 6: Wash your hands after using Enstilar Foam unless you are using the medicine to treat your hands.
Storing Enstilar Foam
- Store Enstilar Foam at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not expose Enstilar Foam to heat or store at temperatures above 120°F (49°C).
- Do not puncture or burn the Enstilar Foam can.
- Do not freeze Enstilar Foam.
Disposing of Enstilar Foam
- Enstilar Foam has an expiration date (exp.) marked on the can. Do not use after this date.Throw away (dispose of) unused Enstilar Foam 6 months after the can has been opened.
Keep Enstilar Foam and all medicines out of the reach of children.
Label
PRINCIPAL DISPLAY PANEL – 60 GRAM CAN CARTON
- NDC 50222-302-60
- Rx only
- Enstilar®
(calcipotriene and
betamethasone
dipropionate)
Foam, 0.005%/0.064% - Shake before Use
- For Topical Use Only
- Not for oral, ophthalmic,
or intravaginal use - Net Wt.
60 gram - LEO®

PRINCIPAL DISPLAY PANEL – 120 GRAM CAN CARTON
- NDC 50222-302-66
Rx only - Enstilar®
(calcipotriene and betamethasone
dipropionate)
Foam, 0.005%/0.064% - Shake before Use
- For Topical Use Only
- Not for oral, ophthalmic, or intravaginal use
- Net Wt. 120 gram
(2 cans of 60 gram) - LEO®

SRC: NLM .
