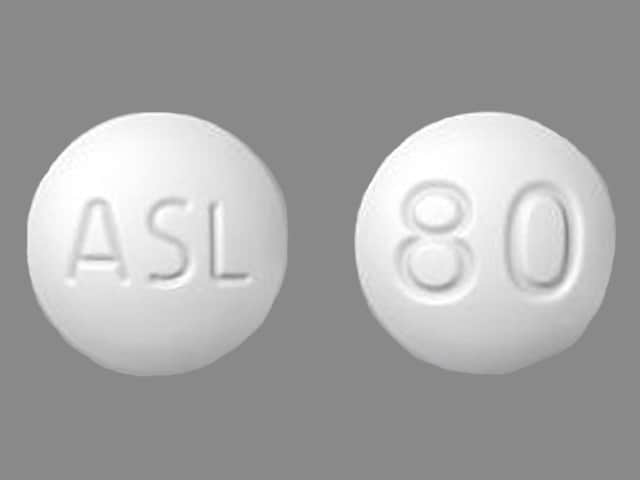
Edarbi
Generic name: azilsartan
Drug class: Angiotensin receptor blockers
Medically reviewed by A Ras MD.
What is Edarbi?
Edarbi is a prescription medicine called an angiotensin II receptor blocker (ARB) used to treat high blood pressure (hypertension) in adults.
Your doctor may prescribe other medicines for you to take along with Edarbi to treat your high blood pressure. It is not known if Edarbi is safe and effective in children under 18 years of age.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too great.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. Edarbi tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure may lower your chance of having a stroke or heart attack.
Description
Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is an angiotensin II receptor blocker.
The drug substance used in the drug product formulation is the potassium salt of azilsartan medoxomil, also known by the US accepted name of azilsartan kamedoxomil and is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2′-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate monopotassium salt.
Its empirical formula is C30H23KN4O8 and its structural formula is:
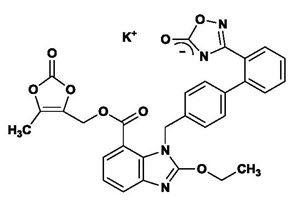
Azilsartan kamedoxomil is a white to nearly white powder with a molecular weight of 606.62. It is practically insoluble in water and freely soluble in methanol.
Edarbi is available for oral use as tablets. The tablets have a characteristic odor. Each Edarbi tablet contains 42.68 or 85.36 mg of azilsartan kamedoxomil, which is equivalent to containing 40 mg or 80 mg respectively, of azilsartan medoxomil and the following inactive ingredients: mannitol, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate.
Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzymes (ACE, kinase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Azilsartan medoxomil is an orally administered prodrug that is rapidly converted by esterases during absorption to the active moiety, azilsartan. Azilsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathway for angiotensin II synthesis.
An AT2 receptor is also found in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Azilsartan has more than a 10,000-fold greater affinity for the AT1 receptor than for the AT2 receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction catalyzed by ACE. Because azilsartan does not inhibit ACE (kinase II), it should not affect bradykinin levels. Whether this difference has clinical relevance is not yet known. Azilsartan does not bind to or block other receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of azilsartan on blood pressure.
What is the most important information I should know about Edarbi?
- Edarbi can cause harm or death to your unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you become pregnant while taking Edarbi, tell your doctor right away. Your doctor may switch you to a different medicine to treat your high blood pressure.
What should I tell my healthcare provider before taking Edarbi?
Before you take Edarbi, tell your doctor if you:
- have been told that you have abnormal body salt (electrolytes) levels in your blood
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Edarbi?”
- are breastfeeding or plan to breastfeed. It is not known if Edarbi passes into your breast milk. You and your doctor should decide if you will take Edarbi or breastfeed. You should not do both. Talk with your doctor about the best way to feed your baby if you take Edarbi.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Especially tell your doctor if you take:
- other medicines used to treat your high blood pressure or heart problem
- water pills (diuretic)
Ask your doctor if you are not sure if you are taking a medicine listed above.
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I take Edarbi?
- Your doctor will tell you how much Edarbi to take and when to take it. Follow his/her instructions.
- Edarbi can be taken with or without food.
- If you take too much Edarbi, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of Edarbi?
Edarbi may cause side effects, including:
- Harm or death to your unborn fetus if taken in the second or third trimester. See “What is the most important information I should know about Edarbi?”
- Low blood pressure (hypotension) and dizziness is most likely to happen if you also:
If you feel faint or dizzy, lie down and call your doctor right away.
These are not all the possible side effects with Edarbi. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Edarbi
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not give Edarbi to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Edarbi. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Edarbi that is written for health professionals.
For more information, go to www.edarbi.com or call 1-866-516-4950.
How should I store Edarbi?
- Store Edarbi at 59°F to 86°F (15°C to 30°C).
- Store Edarbi in the original container that you received from your pharmacist or doctor. Do not put Edarbi into a different container.
- Keep Edarbi in a tightly closed container, and keep Edarbi out of the light.
Keep Edarbi and all medicines out of the reach of children.
What are the ingredients in Edarbi?
Active ingredient: azilsartan medoxomil
Inactive ingredients: mannitol, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate.
Label
PRINCIPAL DISPLAY PANEL – 40 MG TABLET BOTTLE LABEL
- NDC 60631-040-30
30 Tablets - edarbi
(azilsartan medoxomil)
tablets - 40 mg
- Rx Only

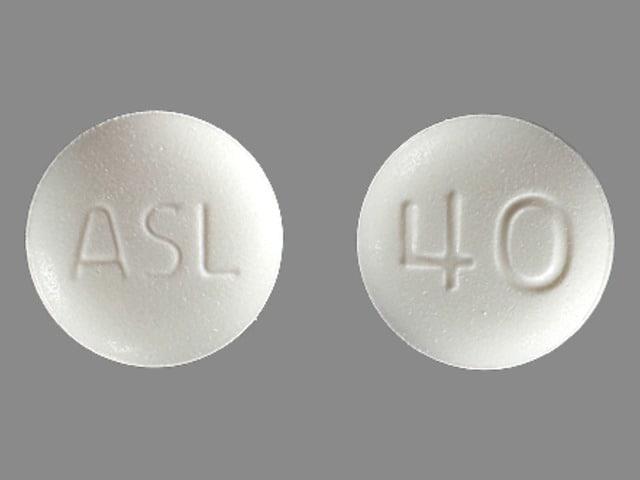
PRINCIPAL DISPLAY PANEL – 80 MG TABLET BOTTLE LABEL