Dulera
Generic name: formoterol and mometasone
Drug class: Bronchodilator combinations
Medically reviewed by A Ras MD.
What is Dulera?
Dulera combines an inhaled corticosteroid medicine (ICS), mometasone furoate, and a long-acting beta2-agonist medicine (LABA), formoterol. ICS medicines such as mometasone furoate help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
LABA medicines such as formoterol help the muscles around the airways in your lungs stay relaxed to prevent asthma symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe. Dulera is not used to relieve sudden breathing problems and will not replace a rescue inhaler.
It is not known if Dulera is safe and effective in children less than 5 years of age.
Dulera is used for asthma as follows Dulera is a prescription medicine used to control symptoms of asthma and prevent symptoms such as wheezing in people 5 years of age and older. Dulera contains formoterol. LABA medicines such as formoterol when used alone increase the risk of hospitalizations and death from asthma problems. Dulera contains an ICS and a LABA. When an ICS and LABA are used together, there is not a significant increased risk in hospitalizations and death from asthma problems. Dulera is not for adults and children with asthma who are well controlled with an asthma control medicine, such as a low to medium dose ICS medicine. Dulera is for adults and children with asthma who need both an ICS and LABA medicine.
Description
DULERA 50 mcg/5 mcg, DULERA 100 mcg/5 mcg, and DULERA 200 mcg/5 mcg are combinations of mometasone furoate and formoterol fumarate dihydrate for oral inhalation only.
One active component of DULERA is mometasone furoate, a corticosteroid having the chemical name 9,21-dichloro-11(Beta),17-dihydroxy-16 (alpha)-methylpregna-1,4-diene-3,20-dione 17-(2-furoate) with the following chemical structure:
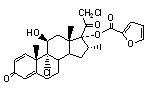
Mometasone furoate is a white powder with an empirical formula of C27H30Cl2O6, and molecular weight 521.44. It is practically insoluble in water; slightly soluble in methanol, ethanol, and isopropanol; soluble in acetone.
One active component of DULERA is formoterol fumarate dihydrate, a racemate. Formoterol fumarate dihydrate is a selective beta2-adrenergic bronchodilator having the chemical name of (±)-2-hydroxy-5-[(1RS)-1-hydroxy-2-[[(1RS)-2-(4-methoxyphenyl)-1-methylethyl]-amino]ethyl]formanilide fumarate dihydrate with the following chemical structure:
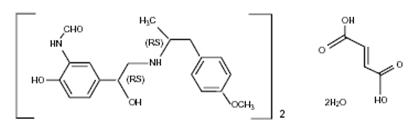
Formoterol fumarate dihydrate has a molecular weight of 840.9, and its empirical formula is (C19H24N2O4)2•C4H4O4•2H2O. Formoterol fumarate dihydrate is a white to yellowish powder, which is freely soluble in glacial acetic acid, soluble in methanol, sparingly soluble in ethanol and isopropanol, slightly soluble in water, and practically insoluble in acetone, ethyl acetate, and diethyl ether.
DULERA 50 mcg/5 mcg, 100 mcg/5 mcg, and 200 mcg/5 mcg are each formulated as a hydrofluoroalkane (HFA-227; 1, 1, 1, 2, 3, 3, 3-heptafluoropropane) propelled pressurized metered dose inhaler containing sufficient amount of drug for 60 or 120 inhalations . After priming, each actuation of the inhaler delivers 60, 115, or 225 mcg of mometasone furoate and 5.5 mcg of formoterol fumarate dihydrate in 69.6 mg of suspension from the valve and delivers 50, 100, or 200 mcg of mometasone furoate and 5 mcg of formoterol fumarate dihydrate from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system. DULERA also contains anhydrous alcohol as a cosolvent and oleic acid as a surfactant.
DULERA should be primed before using for the first time by releasing 4 test sprays into the air, away from the face, shaking well before each spray. In cases where the inhaler has not been used for more than 5 days, prime the inhaler again by releasing 4 test sprays into the air, away from the face, shaking well before each spray.
Mechanism of Action
DULERA: DULERA contains both mometasone furoate and formoterol fumarate; therefore, the mechanisms of actions described below for the individual components apply to DULERA. These drugs represent two different classes of medications (a synthetic corticosteroid and a selective long-acting beta2-adrenergic receptor agonist) that have different effects on clinical, physiological, and inflammatory indices of asthma.
Mometasone furoate: Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on asthma is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of inhibitory effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation and in the asthmatic response. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Mometasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor, which is approximately 12 times that of dexamethasone, 7 times that of triamcinolone acetonide, 5 times that of budesonide, and 1.5 times that of fluticasone. The clinical significance of these findings is unknown.
Formoterol fumarate: Formoterol fumarate is a long-acting selective beta2-adrenergic receptor agonist (beta2-agonist). Inhaled formoterol fumarate acts locally in the lung as a bronchodilator. In vitro studies have shown that formoterol has more than 200-fold greater agonist activity at beta2-receptors than at beta1-receptors. Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including formoterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′, 5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
In vitro tests show that formoterol is an inhibitor of the release of mast cell mediators, such as histamine and leukotrienes, from the human lung. Formoterol also inhibits histamine-induced plasma albumin extravasation in anesthetized guinea pigs and inhibits allergen-induced eosinophil influx in dogs with airway hyper-responsiveness. The relevance of these in vitro and animal findings to humans is unknown.
Who should not take Dulera
Do not use Dulera:
- to treat sudden severe symptoms of asthma.
- as a rescue inhaler.
- if you are allergic to any of the ingredients in Dulera. See the end of this Patient Information guide for a list of ingredients in Dulera.
What should I tell my healthcare provider before taking Dulera?
Before and during treatment with Dulera, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have high blood pressure.
- have seizures.
- have thyroid problems.
- have diabetes.
- have liver problems.
- have osteoporosis.
- have an immune system problem.
- have eye problems such as increased pressure in the eye, glaucoma, cataracts, blurred vision, or other changes in your vision.
- are allergic to any medicines.
- are exposed to chickenpox or measles.
- have an aneurysm (swelling of an artery).
- have a pheochromocytoma (a tumor of the adrenal gland that can affect your blood pressure).
- are scheduled to have surgery.
- are pregnant or planning to become pregnant. It is not known if Dulera may harm your unborn baby.
- are breastfeeding. It is not known if Dulera passes into your milk and if it can harm your baby. You and your healthcare provider should decide if you will take Dulera while breastfeeding.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. Dulera and certain other medicines may interact with each other. This may cause serious side effects.
Especially, tell your healthcare provider if you take antifungal medicines, such as ketoconazole, or anti-HIV medicines, such as ritonavir and cobicistat-containing products. The anti-HIV medicines norvir (ritonavir capsules) soft gelatin, norvir (ritonavir oral solution), and kaletra (lopinavir/ritonavir) Tablets contain ritonavir.
For some medicines (including medicines for HIV such as ritonavir, cobicistat-containing products, and certain antifungals and antibiotics) your doctor may wish to monitor you carefully.
Know the medicines you take. Keep a list and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I use Dulera?
See the step-by-step instructions for using Dulera that come with your medication. Do not use Dulera unless your healthcare provider has taught you and you understand how to use it. Ask your healthcare provider or pharmacist if you have any questions.
- Use Dulera exactly as prescribed. Do not use Dulera more often than prescribed. Dulera comes in 3 strengths. Your healthcare provider has prescribed the strength that is best for you. Note the differences between Dulera and your other inhaled medicines, including the differences in prescribed use and physical appearance.
- For children aged 5 to less than 12 years, use Dulera 50 mcg/5 mcg.
- For adults and adolescents 12 years of age and older, use Dulera 100 mcg/5 mcg or Dulera 200 mcg/5 mcg.
- Dulera should be taken every day as 2 puffs in the morning and 2 puffs in the evening.
- If you miss a dose of Dulera, skip your missed dose and take your next dose at your regular time. Do not take Dulera more often or use more puffs than you have been prescribed.
- While you are using Dulera 2 times each day, do not use other medicines that contain a long-acting beta2-agonist (LABA) for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA medicines.
- If you take more Dulera than your healthcare provider has prescribed, get medical help right away if you have any unusual symptoms, such as problems breathing, palpitations, chest pain, increased heart rate, nervousness or shakiness.
- Do not change or stop using Dulera or other asthma medicines used to control or treat your breathing problems unless told to do so by your healthcare provider. Your healthcare provider will change your medicines as needed.
- Dulera does not relieve sudden asthma symptoms. Always have a rescue inhaler with you to treat sudden symptoms. Use your rescue inhaler if you have breathing problems between doses of Dulera. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Remove the cap from the mouthpiece of the actuator before using Dulera.
- Do not remove the canister from the actuator because:
- You may not receive the correct amount of medicine.
- The dose counter may not function properly.
- Reinsertion may cause the dose counter to count down by 1 and may discharge a puff.
- After each dose (2 puffs) of Dulera, rinse your mouth with water. Spit out the water. Do not swallow it. This will help to lessen the chance of getting a yeast infection (thrush) in the mouth and throat.
- Prime (sprays released into the air before use) your inhaler away from your face. Do not spray Dulera in your eyes. If you accidentally get Dulera in your eyes, rinse your eyes with water and if redness or irritation continues, call your healthcare provider.
- Call your healthcare provider or get medical care right away if:
- your breathing problems worsen with Dulera
- you need to use your rescue inhaler more often than usual
- your rescue inhaler does not work as well for you at relieving symptoms
- you need to use 4 or more inhalations of your rescue inhaler for 2 or more days in a row
- you use 1 whole canister of your rescue inhaler in 4 weeks’ time
- your peak flow meter results decrease. Your healthcare provider will tell you the numbers that are right for you.
- your asthma symptoms do not improve after using Dulera regularly for 2 weeks
What are the possible side effects of Dulera?
Dulera may cause serious side effects, including:
- Thrush in the mouth and throat. You may develop thrush, a yeast infection (Candida albicans), in your mouth or throat. After each dose of Dulera (2 puffs), rinse your mouth with water. Spit out the water. Do not swallow it. This will help to prevent thrush in your mouth or throat.
- Immune system effects and a higher chance for infections. Signs of infection may include:
- Adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines and start inhaled corticosteroid medicines.
- Increased wheezing right after taking Dulera. Always have a rescue inhaler with you to treat sudden wheezing.
- Serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- rash
- swelling, including swelling of the face, mouth, and tongue
- hives
- breathing problems
- Using too much of a LABA medicine may cause:
- chest pain
- a fast and irregular heartbeat
- tremor
- dizziness
- seizures
- increased or decreased blood pressure
- headache
- nervousness
- weakness
- electrocardiogram (ECG) changes
- Lower bone mineral density. This may be a problem for people who already have a higher chance for low bone density (osteoporosis).
- Slowed growth in children. A child’s growth should be checked often.
- Eye problems including glaucoma, cataracts, and blurred vision. You should have regular eye exams while using Dulera.
- Decreases in blood potassium levels (hypokalemia).
- Increases in blood sugar levels (hyperglycemia).
The most common side effects reported while using Dulera include:
- inflammation of the nose and throat (nasopharyngitis)
- inflammation of the sinuses (sinusitis)
- headache
- flu
- upper respiratory infection
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the side effects of Dulera. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231.
General Information about the safe and effective use of Dulera.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Dulera for a condition for which it was not prescribed. Do not give your Dulera to other people, even if they have the same condition. It may harm them.
You can ask your healthcare provider or pharmacist for information about Dulera that was written for healthcare professionals.
How should I store Dulera?
- Store Dulera at room temperature between 68°F to 77°F (20°C to 25°C).
- The 120-actuation inhaler can be stored in any position. For the 60-actuation inhaler, after priming, store the inhaler with the mouthpiece down or sideways.
- The contents of your Dulera are under pressure. Do not puncture. Do not use or store near heat or open flame. Storage above 120°F may cause the canister to burst.
- Do not throw container into fire or incinerator.
- Keep Dulera and all medicines out of the reach of children.
What are the ingredients in Dulera?
Active ingredients: mometasone furoate and formoterol fumarate dihydrate
Inactive ingredients: hydrofluoroalkane (HFA-227), anhydrous alcohol and oleic acid\
Label
PRINCIPAL DISPLAY PANEL – 50 MCG/5 MCG CANISTER CARTON
- NDC 78206-125-01
- Dulera®
(mometasone furoate and
formoterol fumarate dihydrate)
Inhalation Aerosol - 50 mcg/5 mcg
per actuation - For oral inhalation only
- SHAKE WELL BEFORE USING.
- Dulera canister to be used with Dulera actuator only.
- Rx only
- 120 Metered Actuations
Net Wt. 13g

SRC: NLM .
