Dalfampridine
Generic name: dalfampridine
Brand name: Ampyra
Dosage form: oral tablet, extended release (10 mg)
Drug class: Miscellaneous central nervous system agents
Medically reviewed by A Ras MD.
What is dalfampridine?
Dalfampridine is a prescription medicine that is used to treat MS (multiple sclerosis).
Description
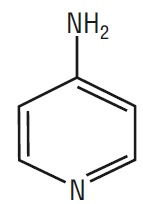
Dalfampridine extended-release tablet is a potassium channel blocker, available in a 10 mg tablet strength. Each tablet contains 10 mg dalfampridine, formulated as an extended-release tablet for twice-daily oral administration. Dalfampridine is also known by its chemical name, 4-aminopyridine, with the following structure:
Dalfampridine extended-release tablets are available in a 10 mg strength and are white to off-white colored oval shaped biconvex film coated tablet debossed with “C51” on one side and plain on other side. Tablet dimensions are approximately 13 mm (Length) and 8 mm (Width). Inactive ingredients consist of hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycols, silicon dioxide and titanium dioxide.
Dalfampridine is a fine white to off-white powder with a molecular weight of 94.1, CAS 504-24-5, and a molecular formula of C 5H 6N 2. At ambient conditions, dalfampridine is soluble in water, methanol, acetone, tetrahydrofuran, isopropanol, acetonitrile, N,N-dimethylformamide, dimethylsulfoxide, and ethanol.
Mechanism of action
The mechanism by which dalfampridine exerts its therapeutic effect has not been fully elucidated. Dalfampridine is a broad spectrum potassium channel blocker. In animal studies, dalfampridine has been shown to increase conduction of action potentials in demyelinated axons through inhibition of potassium channels.
Before taking dalfampridine, tell your doctor:
- If you are allergic to dalfampridine; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have ever had a seizure.
- If you have kidney disease.
- If you are taking or will be taking another drug like this one.
This is not a list of all drugs or health problems that interact with dalfampridine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take dalfampridine with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take dalfampridine?
- Tell all of your health care providers that you take dalfampridine. This includes your doctors, nurses, pharmacists, and dentists.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- This medicine may cause seizures in some patients. Talk with the doctor.
- If you are 65 or older, use dalfampridine with care. You could have more side effects.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is dalfampridine best taken?
Use dalfampridine as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take with or without food.
- Swallow whole with fluid. Do not chew, break, crush, or dissolve
- If you have trouble swallowing, talk with your doctor.
- Do not take dalfampridine more often than you are told. This may raise the risk of seizures. Be sure you know how far apart to take your doses.
What do I do if I miss a dose?
- Skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of dalfampridine that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of a urinary tract infection (UTI) like blood in the urine, burning or pain when passing urine, feeling the need to pass urine often or right away, fever, lower stomach pain, or pelvic pain.
- Very bad dizziness or passing out.
- Seizures.
- Change in balance.
- Shortness of breath.
- A burning, numbness, or tingling feeling that is not normal.
What are some other side effects of dalfampridine?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Upset stomach.
- Feeling dizzy, tired, or weak.
- Headache.
- Trouble sleeping.
- Back pain.
- Nose or throat irritation.
- Constipation.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out dalfampridine?
- Store at room temperature in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
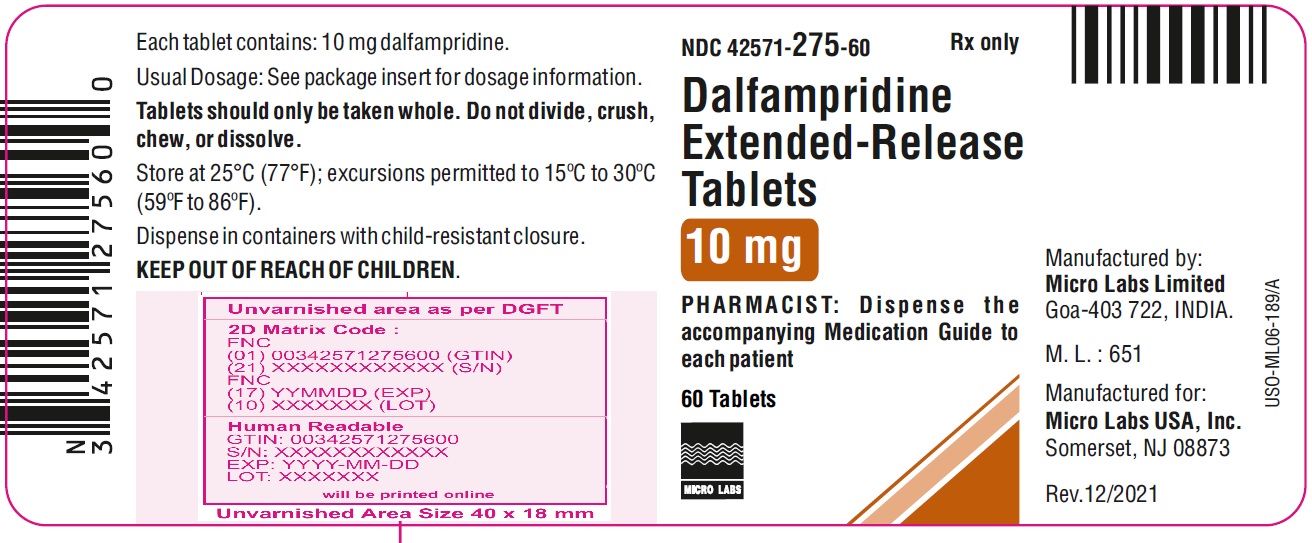
Label
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- NDC 42571-275-60
Rx only
Dalfampridine Extended-Release Tablets
10 mg
PHARMACIST: Dispense the
accompanying Medication Guide to
each patient
60 Tablets
MICRO LABS LIMITED

SRC: NLM .
