Cosentyx
Generic name: secukinumab
What is Cosentyx?
Cosentyx is a prescription medicine used to treat adults:
- with moderate to severe plaque psoriasis that involves large areas or many areas of the body, and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light alone or with systemic therapy)
- with active psoriatic arthritis
- with active ankylosing spondylitis
- with active non-radiographic axial spondyloarthritis and objective signs of inflammation
Cosentyx may improve your psoriasis, psoriatic arthritis, ankylosing spondylitis and non-radiographic axial spondyloarthritis but it may also lower the ability of your immune system to fight infections.
It is not known if Cosentyx is safe and effective in children.
Description
Secukinumab, a recombinant human monoclonal IgG1/κ antibody, is an interleukin-17A antagonist. It is expressed in a recombinant Chinese Hamster Ovary (CHO) cell line. Secukinumab has a molecular mass of approximately 151 kDa; both heavy chains of secukinumab contain oligosaccharide chains.
Mechanism of Action
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Secukinumab inhibits the release of proinflammatory cytokines and chemokines.
What is the most important information I should know about Cosentyx?
Cosentyx is a medicine that affects your immune system. Cosentyx may increase your risk of having serious side effects such as:
Infections. Cosentyx may lower the ability of your immune system to fight infections and may increase your risk of infections.
- Your healthcare provider should check you for tuberculosis (TB) before starting treatment with Cosentyx.
- If your healthcare provider feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with Cosentyx and during treatment with Cosentyx.
- Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with Cosentyx. Do not take Cosentyx if you have an active TB infection.
Before starting Cosentyx, tell your healthcare provider if you:
- are being treated for an infection
- have an infection that does not go away or that keeps coming back
- have TB or have been in close contact with someone with TB
- think you have an infection or have symptoms of an infection such as:
- fever, sweats, or chills
- muscle aches
- cough
- shortness of breath
- blood in your phlegm
- weight loss
- warm, red, or painful skin or sores on your body
- diarrhea or stomach pain
- burning when you urinate or urinate more often than normal
After starting Cosentyx, call your healthcare provider right away if you have any of the signs of infection listed above. Do not use Cosentyx if you have any signs of infection unless you are instructed to by your healthcare provider.
See “What are the possible side effects of Cosentyx?” for more information about side effects.
Who should not take Cosentyx?
Do not use Cosentyx if you have had a severe allergic reaction to secukinumab or any of the other ingredients in Cosentyx. See below for a complete list of ingredients in Cosentyx.
What should I tell my healthcare provider before taking Cosentyx?
Before taking Cosentyx, tell your healthcare provider about all of your medical conditions, including if you: have any of the conditions or symptoms listed in the section “What is the most important information I should know about Cosentyx?”
- have inflammatory bowel disease (Crohn’s disease or ulcerative colitis)
- are allergic to latex. The needle cap on the Cosentyx Sensoready pen and prefilled syringe contains latex.
- have recently received or are scheduled to receive an immunization (vaccine). People who take Cosentyx should not receive live vaccines.
- are pregnant or plan to become pregnant. It is not known if Cosentyx can harm your unborn baby. You and your healthcare provider should decide if you will use Cosentyx.
- are breastfeeding or plan to breastfeed. It is not known if Cosentyx passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Cosentyx?
See the detailed “Instructions for use” that comes with your Cosentyx for information on how to prepare and inject a dose of Cosentyx, and how to properly throw away (dispose of) used Cosentyx Sensoready pens and prefilled syringes.
- Use Cosentyx exactly as prescribed by your healthcare provider.
- If your healthcare provider decides that you or a caregiver may give your injections of Cosentyx at home, you should receive training on the right way to prepare and inject Cosentyx. Do not try to inject Cosentyx yourself, until you or your caregiver has been shown how to inject Cosentyx by your healthcare provider.
- Cosentyx comes in a Sensoready pen or prefilled syringe that you or your caregiver may use at home to give injections. Your healthcare provider will decide which type of Cosentyx is best for you to use at home.
- Your healthcare provider will prescribe the dose of Cosentyx that is right for you.
- If your prescribed dose of Cosentyx is 150 mg, you must give 1 injection of Cosentyx for each dose.
- If your prescribed dose of Cosentyx is 300 mg, you must give 2 injections for each dose.
- Cosentyx is given as an injection under your skin (subcutaneous injection), in your upper legs (thighs) or stomach-area (abdomen) by you or a caregiver. A caregiver may also give you an injection of Cosentyx in your upper outer arm.
- Do not give an injection in an area of the skin that is tender, bruised, red or hard, or in an area of skin that is affected by psoriasis.
- Each injection should be given at a different site. Do not use the 2-inch area around your navel (belly button).
- If you inject more Cosentyx than prescribed, call your healthcare provider or go to the nearest emergency room right away.
What are the possible side effects of Cosentyx?
Cosentyx may cause serious side effects including:
- See “What is the most important information I should know about Cosentyx?”.
- Inflammatory bowel disease. New cases of inflammatory bowel disease or “flare-ups” can happen with Cosentyx, and can sometimes be serious. If you have inflammatory bowel disease (ulcerative colitis or Crohn’s disease), tell your healthcare provider if you have worsening disease symptoms during treatment with Cosentyx or develop new symptoms of stomach pain or diarrhea.
- Serious allergic reactions. Get emergency medical help right away if you get any of the following symptoms of a serious allergic reaction:
- feel faint
- swelling of your face, eyelids, lips, mouth, tongue, or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash
If you have a severe allergic reaction, do not give another injection of Cosentyx.
The most common side effects of Cosentyx include:
- cold symptoms
- diarrhea
- upper respiratory infections
These are not all of the possible side effects of Cosentyx.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Cosentyx
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Cosentyx for a condition for which it was not prescribed. Do not give Cosentyx to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Cosentyx that is written for health professionals.
How should I store Cosentyx?
- Store Cosentyx in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep Cosentyx in the original carton until ready for use to protect from light.
- Do not freeze Cosentyx.
- Do not shake Cosentyx.
Keep Cosentyx and all medicines out of the reach of children.
What are the ingredients in Cosentyx?
Active ingredient: secukinumab
Inactive ingredients:
Sensoready pen and prefilled syringe: L-histidine/histidine hydrochloride monohydrate, L-methionine, polysorbate 80, trehalose dihydrate, and sterile water for injection.
Vial: L-histidine/histidine hydrochloride monohydrate, polysorbate 80, and sucrose.
For more information, call 1-888-669-6682 or go to www.COSENTYX.com
Instruction for use
COSENTYX® (secukinumab)
For Injection
The following information is intended for medical or healthcare professionals only.
IMPORTANT:
- The single-dose vial contains 150 mg of COSENTYX for reconstitution with Sterile Water for Injection (SWFI). Do not use the vial after the expiry date shown on the outer box or vial. If it has expired, return the entire pack to the pharmacy.
- The preparation of the solution for subcutaneous injection shall be done without interruption ensuring that aseptic technique is used. The preparation time from piercing the stopper until end of reconstitution on average takes 20 minutes and should not exceed 90 minutes.
- Throw away (dispose of) the used syringe right away after use. Do not re-use a syringe. See “How should I dispose of a used syringe?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store the vial of COSENTYX in the refrigerator between 2°C to 8°C (36°F to 46°F).
To prepare COSENTYX 150 mg for injection, please adhere to the following instructions:
Instructions for reconstitution of COSENTYX 150 mg for injection:
| Step 1. Remove the vial of COSENTYX 150 mg for injection from the refrigerator and allow to stand for 15 to 30 minutes to reach room temperature. Ensure the Sterile Water for Injection (SWFI) is at room temperature.
Step 2. Reconstitute the lyophilized powder by slowly injecting 1 mL of Sterile Water for Injection (SWFI) into the vial. Direct the stream of SWFI onto the lyophilized powder (See Figure A). |
Figure A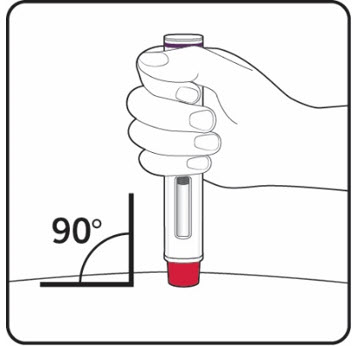 |
| Step 3. Tilt the vial to an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial (See Figure B).
Step 4. Keep the vial standing at room temperature for a minimum of 10 minutes to allow for dissolution. Note that foaming of the solution may occur. Step 5. Tilt the vial to an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial (See Figure B). Step 6. Allow the vial to stand undisturbed at room temperature for approximately 5 minutes. The resulting solution should be clear. Its color may vary from colorless to slightly yellow. Do not use if the lyophilized powder has not fully dissolved or if the liquid contains visible particles, is cloudy or is discolored. Step 7. Prepare the required number of vials (1 vial for the 150 mg dose or 2 vials for the 300 mg dose). |
Figure B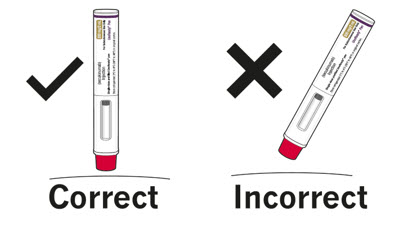 |
| After preparation, use the solution for subcutaneous injection immediately or store at 2°C to 8°C (36°F to 46°F) for up to 24 hours. Do not freeze. After storage at 2°C to 8°C (36°F to 46°F), allow the reconstituted solution to come to room temperature (15 to 30 minutes) before administration. Administer the solution within 1 hour after removal from the 2°C to 8°C (36°F to 46°F) storage. | |
Instructions for administration of COSENTYX solution: |
|
| Step 1. Tilt the vial to an angle of approximately 45 degrees and position the needle tip at the very bottom of the solution in the vial when drawing the solution into the syringe. DO NOT invert the vial.
Step 2. Carefully withdraw slightly more than 1 mL of the solution for subcutaneous injection from the vial into a 1 mL graduated disposable syringe using a suitable needle (e.g., 21G x 2”) (See Figure C). This needle will only be used for withdrawing COSENTYX into the disposable syringe. Prepare the required number of syringes (1 syringe for the 150 mg dose or 2 syringes for the 300 mg dose). |
Figure C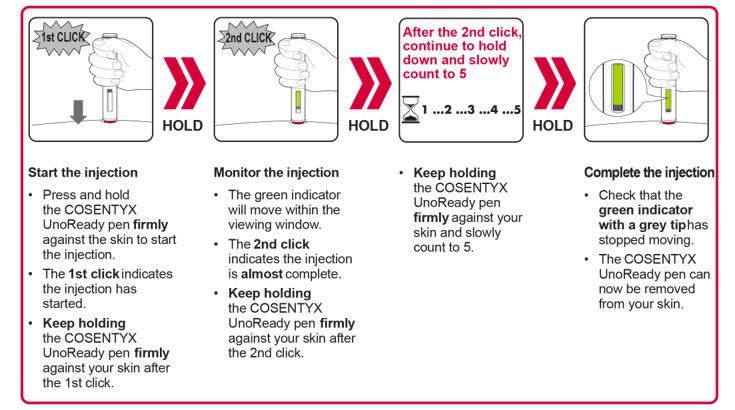 |
| Step 3. With the needle pointing upward, gently tap the syringe to move any air bubbles to the top (See Figure D). | Figure D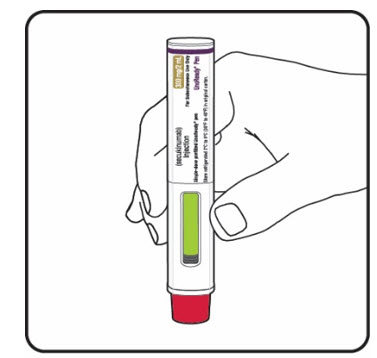 |
| Step 4. Replace the attached needle with a 27G x ½” needle (See Figure E).
Step 5. Expel the air bubbles and advance the plunger to the 1 mL mark. Step 6. Clean the injection site with an alcohol wipe. |
Figure E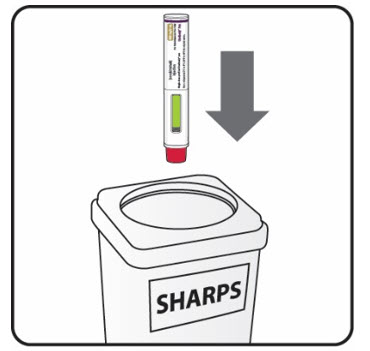 |
| Step 7. Inject the COSENTYX solution subcutaneously into the front of thighs, lower abdomen [but not the area 2 inches around the navel (belly button)] or outer upper arms (See Figure F). Choose a different site each time an injection is administered. Do not inject into areas where the skin is tender, bruised, red, scaly or hard, or in an area of skin that is affected by psoriasis. Avoid areas with scars or stretch marks. | Figure F |
|
How should I dispose of a used syringe? |
|
| Any remaining solution in the vial must not be used and must be discarded in accordance with local requirements. Vials are for single use only. | |
| Put the used syringes and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the syringes and needles in your household trash. | |
| If you do not have an FDA-cleared sharps disposal container, you may use a household container that is: | |
|
|
| When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL. | |
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | |
| Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 US License Number 1244 |
|
| Issued: December 2021 | |
| © Novartis | |
| T2021-164 | |
150 mg/mL single-dose Prefilled Syringe
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the prefilled syringe before you use it for the first time. Children should not inject COSENTYX themselves using the prefilled syringe. An adult caregiver should prepare and inject COSENTYX after receiving proper training in subcutaneous injection technique. Talk to your healthcare provider if you have any questions.
Important:
- Do not use the COSENTYX prefilled syringe if either the seal on the outside carton or the seal of the blister are broken. Keep the COSENTYX prefilled syringe in the sealed carton until you are ready to use it.
- Do not shake the COSENTYX prefilled syringe.
- The needle caps of the prefilled syringes contain latex. Do not handle the prefilled syringes if you are sensitive to latex.
- The prefilled syringe has a needle guard that will be activated to cover the needle after the injection is finished. The needle guard will help to prevent needle stick injuries to anyone who handles the prefilled syringe.
- Do not remove the needle cap until just before you give the injection.
- Avoid touching the syringe guard wings before use. Touching them may cause the syringe guard to be activated too early.
- Throw away (dispose of) the used COSENTYX prefilled syringe right away after use. Do not re-use a COSENTYX prefilled syringe. See “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX prefilled syringes in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep COSENTYX prefilled syringes in the original carton until ready to use to protect from light.
- COSENTYX prefilled syringes may be stored at room temperature, not to exceed temperatures above 86°F (30°C), for up to 4 days.
- Write the date COSENTYX prefilled syringes was removed from the refrigerator in the space provided on the carton.
- If unused and not stored above 30°C (86°F), COSENTYX prefilled syringes may be returned to the refrigerator.
- Throw away COSENTYX if it has been kept outside of the refrigerator and not been used in over 4 days.
- Do not freeze COSENTYX prefilled syringes.
- Throw away (dispose of) any unused COSENTYX prefilled syringes.
Keep COSENTYX and all medicines out of the reach of children.
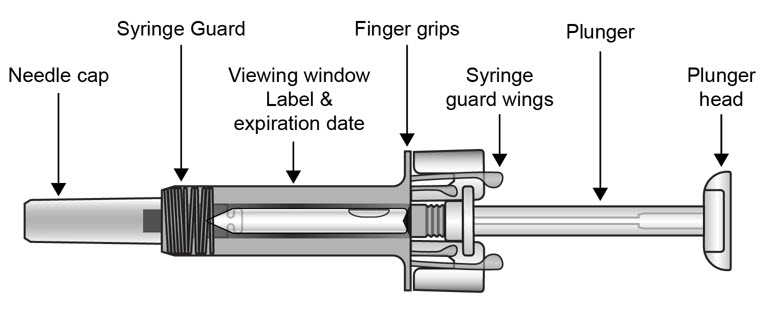
| COSENTYX prefilled syringe parts (see Figure A):
|
|
| Figure A | |
 |
|
| What you need for your injection:
|
|
| Included in the carton: | |
| A new COSENTYX prefilled syringe. | |
Each COSENTYX prefilled syringe contains 150 mg of COSENTYX.
|
|
| Not included in the carton (see Figure B): • 1 Alcohol wipe • 1 Cotton ball or gauze • Sharps disposal containerSee “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use. |
Figure B |
| Prepare the COSENTYX 150 mg prefilled syringe | |
| Step 1. Find a clean, well-lit, flat work surface. | |
| Step 2. Take the carton containing the COSENTYX prefilled syringe out of the refrigerator and leave it unopened on your work surface for about 15 to 30 minutes so that it reaches room temperature. | |
| Step 3. Wash your hands well with soap and water. | |
| Step 4. Remove the COSENTYX prefilled syringe from the outer carton and take it out of the blister. | |
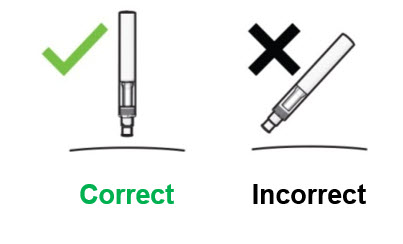
| Step 5. Look through the viewing window on the COSENTYX prefilled syringe. The liquid inside should be clear. The color may be colorless to slightly yellow. You may see a small air bubble in the liquid. This is normal. Do not use the prefilled syringe if the liquid contains visible particles, or if the liquid is cloudy or discolored. | |
| Step 6. Do not use the COSENTYX prefilled syringe if it is broken. Return the prefilled syringe and the package it came in to the pharmacy. | |
| Step 7. Do not use the COSENTYX prefilled syringe if the expiration date has passed. | |
| Choose and clean the injection site | |
|
Figure C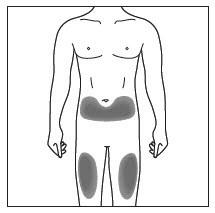 |
| Step 8. Using a circular motion, clean the injection site with the alcohol wipe. Leave it to dry before injecting. Do not touch the cleaned area again before injecting. | Figure D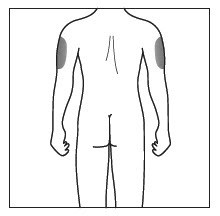 |
Giving the injection |
|
| Step 9. Carefully remove the needle cap from the COSENTYX prefilled syringe (see Figure E). Throw away the needle cap. You may see a drop of liquid at the end of the needle. This is normal. | Figure E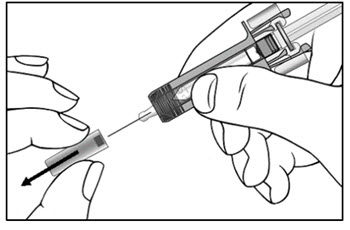 |
| Step 10. With one hand gently pinch the skin at the injection site. With your other hand insert the needle into your skin at a 45-degree angle as shown (see Figure F). Push the needle all the way in to make sure that you inject your full dose. | Figure F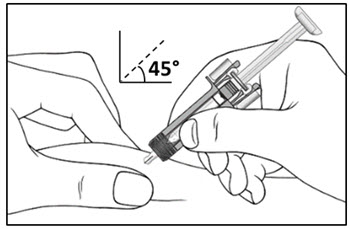 |
| Step 11. Hold the COSENTYX prefilled syringe finger grips as shown (see Figure G). Slowly press down on the plunger as far as it will go, so that the plunger head is completely between the syringe guard wings. This will make sure that the syringe guard has been activated.
Step 12. Continue to press fully on the plunger for an additional 5 seconds. Hold the syringe in place for the full 5 seconds. |
Figure G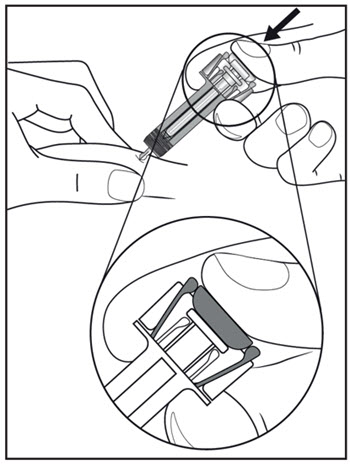 |
| Step 13. Keep the plunger fully depressed while you carefully pull the needle straight out from the injection site (see Figure H). | Figure H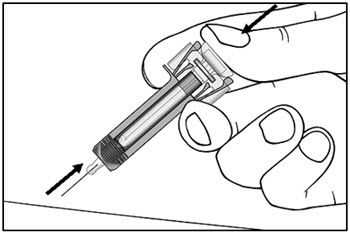 |
| Step 14. Slowly release the plunger and allow the syringe guard to automatically cover the exposed needle (see Figure I).
Step 15. There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. |
Figure I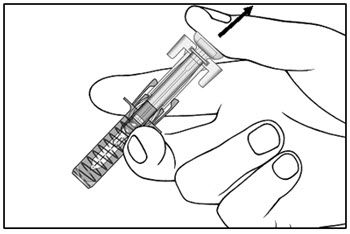 |
If your prescribed dose of COSENTYX is 300 mg, repeat steps 4 through 15 with a new COSENTYX prefilled syringe.How should I dispose of used COSENTYX prefilled syringes? |
|
| Step 16. Put your used prefilled syringes in a FDA-cleared sharps disposal container right away after use (see Figure J). Do not throw away (dispose of) prefilled syringes in your household trash.If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
a. made of a heavy-duty plastic, b. can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out, c. upright and stable during use, d. leak-resistant, and e. properly labeled to warn of hazardous waste inside the container. When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes and prefilled syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL. |
Figure J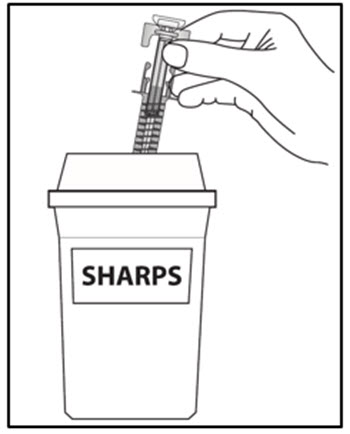 |
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: December 2021 |
|
| Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 US License Number 1244 |
|
| © Novartis | |
| T2021-165 | |
75 mg/0.5 mL single-dose Prefilled Syringe
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the prefilled syringe before you use it for the first time. Children should not inject COSENTYX themselves using the prefilled syringe. An adult caregiver should prepare and inject COSENTYX after receiving proper training in subcutaneous injection technique. Talk to your healthcare provider if you have any questions.
Important:
- Do not use the COSENTYX prefilled syringe if either the seal on the outside carton or the seal of the blister are broken. Keep the COSENTYX prefilled syringe in the sealed carton until you are ready to use it.
- Inject COSENTYX within 1 hour after taking it out of the refrigerator.
- Do not shake the COSENTYX prefilled syringe.
- The needle cap of the prefilled syringe contains latex. Do not handle the prefilled syringe if you are sensitive to latex.
- The prefilled syringe has a needle guard that will be activated to cover the needle after the injection is finished. The needle guard will help to prevent needle stick injuries to anyone who handles the prefilled syringe.
- Do not remove the needle cap until just before you give the injection.
- Avoid touching the syringe guard wings before use. Touching them may cause the syringe guard to be activated too early.
- Throw away (dispose of) the used COSENTYX prefilled syringe right away after use. Do not re-use a COSENTYX prefilled syringe. See “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX prefilled syringe in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep COSENTYX prefilled syringe in the original carton until ready to use to protect from light.
- Do not freeze COSENTYX prefilled syringe.
- Throw away (dispose of) any unused COSENTYX prefilled syringe.
Keep COSENTYX and all medicines out of the reach of children.
| COSENTYX prefilled syringe parts (see Figure A):
|
|
| Figure A | |
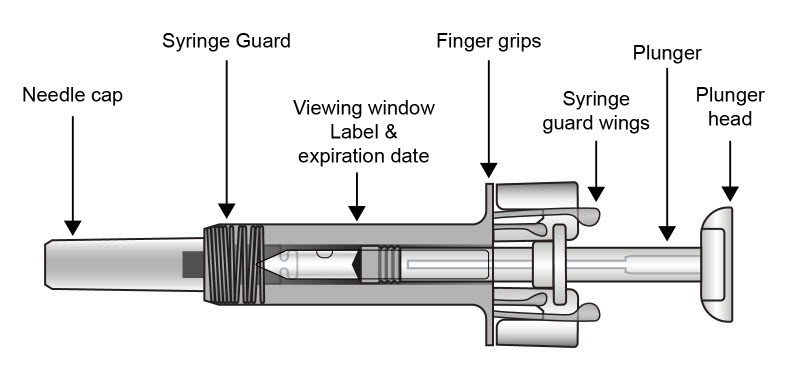 |
|
| What you need for your injection:
|
|
| Included in the carton: | |
| A new COSENTYX prefilled syringe. | |
| Each COSENTYX prefilled syringe contains 75 mg of COSENTYX. | |
| Not included in the carton (see Figure B): • 1 Alcohol wipe • 1 Cotton ball or gauze • Sharps disposal containerSee “How should I dispose of used COSENTYX prefilled syringes?” at the end of this Instructions for Use. |
Figure B |
| Prepare the COSENTYX 75 mg Prefilled Syringe | |
| Step 1. Find a clean, well-lit, flat work surface. | |
| Step 2. Take the carton containing the COSENTYX prefilled syringe out of the refrigerator and leave it unopened on your work surface for about 15 to 30 minutes so that it reaches room temperature. | |
| Step 3. Wash your hands well with soap and water. | |
| Step 4. Remove the COSENTYX prefilled syringe from the outer carton and take it out of the blister. | |
| Step 5. Look through the viewing window on the COSENTYX prefilled syringe. The liquid inside should be clear. The color may be colorless to slightly yellow. You may see a small air bubble in the liquid. This is normal. Do not use the prefilled syringe if the liquid contains visible particles, or if the liquid is cloudy or discolored. | |
| Step 6. Do not use the COSENTYX prefilled syringe if it is broken. Return the prefilled syringe and the package it came in to the pharmacy. | |
| Step 7. Do not use the COSENTYX prefilled syringe if the expiration date has passed. | |
| Choose and clean the injection site | |
|
Figure C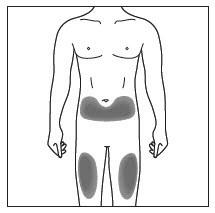 |
| Step 8. Using a circular motion, clean the injection site with the alcohol wipe. Leave it to dry before injecting. Do not touch the cleaned area again before injecting. | Figure D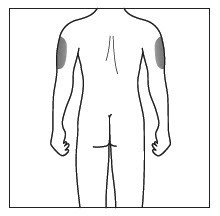 |
Giving the injection |
|
| Step 9. Carefully remove the needle cap from the COSENTYX prefilled syringe (see Figure E). Throw away the needle cap. You may see a drop of liquid at the end of the needle. This is normal. | Figure E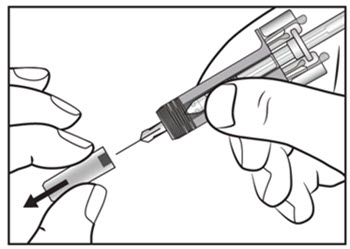 |
| Step 10. With one hand gently pinch the skin at the injection site. With your other hand insert the needle into your skin at a 45-degree angle as shown (see Figure F). Push the needle all the way in to make sure that you inject your full dose. | Figure F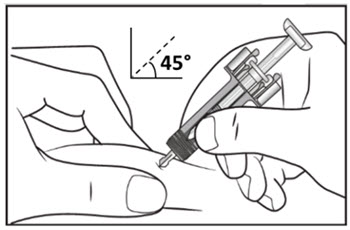 |
| Step 11. Hold the COSENTYX prefilled syringe finger grips as shown (see Figure G). Slowly press down on the plunger as far as it will go, so that the plunger head is completely between the syringe guard wings. This will make sure that the syringe guard has been activated.
Step 12. Continue to press fully on the plunger for an additional 5 seconds. Hold the syringe in place for the full 5 seconds. |
Figure G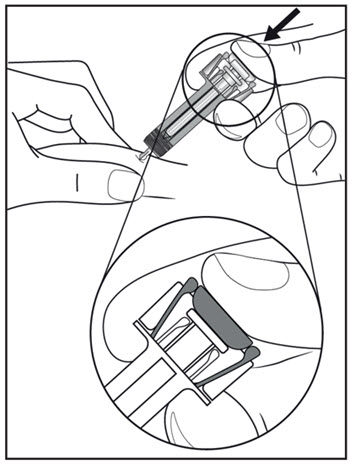 |
| Step 13. Keep the plunger fully depressed while you carefully pull the needle straight out from the injection site (see Figure H). | Figure H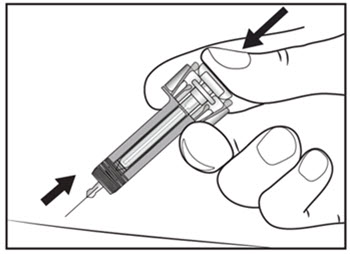 |
| Step 14. Slowly release the plunger and allow the syringe guard to automatically cover the exposed needle (see Figure I).
Step 15. There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. |
Figure I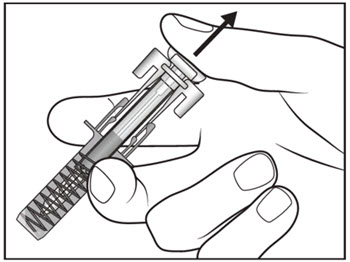 |
| How should I dispose of used COSENTYX prefilled syringe? Step 16. Put your used prefilled syringe in a FDA-cleared sharps disposal container right away after use (see Figure J). Do not throw away (dispose of) prefilled syringes in your household trash.If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes and prefilled syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL. |
Figure J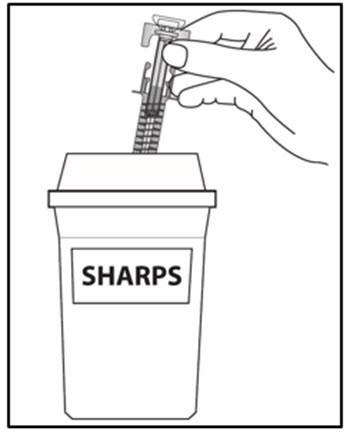 |
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: December 2021 |
|
| Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 US License Number 1244 |
|
| © Novartis | |
| T2021-166 | |
150 mg/mL single-dose Sensoready® Pen
Be sure that you read, understand, and follow this Instructions for Use before injecting COSENTYX. Your healthcare provider should show you how to prepare and inject COSENTYX properly using the Sensoready Pen before you use it for the first time. Children should not inject COSENTYX themselves using the Sensoready pen. An adult caregiver should prepare and inject COSENTYX after receiving proper training in subcutaneous injection technique. Talk to your healthcare provider if you have any questions.
Important:
- Do not use the COSENTYX Sensoready Pen if either the seal on the outer carton or the seal on the pen is broken. Keep the COSENTYX Sensoready Pen in the sealed outer carton until you are ready to use it.
- Do not shake the COSENTYX Sensoready Pen.
- The caps of the Sensoready Pens contain latex. Do not handle the Sensoready Pens if you are sensitive to latex.
- If you drop your COSENTYX Sensoready Pen, do not use it if the Sensoready Pen looks damaged, or if you dropped it with the cap removed.
- Throw away (dispose of) the used COSENTYX Sensoready Pen right away after use. Do not re-use a COSENTYX Sensoready Pen. See “How should I dispose of used COSENTYX Sensoready Pens?” at the end of this Instructions for Use.
How should I store COSENTYX?
- Store your carton of COSENTYX Sensoready Pens in a refrigerator, between 36°F to 46°F (2°C to 8°C).
- Keep COSENTYX Sensoready Pens in the original carton until ready to use to protect from light.
- COSENTYX Sensoready pens may be stored at room temperature, not to exceed temperatures above 86°F (30°C), for up to 4 days.
- Write the date COSENTYX Sensoready pens was removed from the refrigerator in the space provided on the carton.
- If unused and not stored above 30°C (86°F), COSENTYX Sensoready pens may be returned to the refrigerator.
- Throw away COSENTYX if it has been kept outside of the refrigerator and not been used in over 4 days.
- Do not freeze COSENTYX Sensoready Pens.
- Throw away (dispose of) any unused COSENTYX Sensoready Pens.
Keep COSENTYX and all medicines out of the reach of children.
| COSENTYX Sensoready Pen parts (see Figure A): | |
| Figure A | |
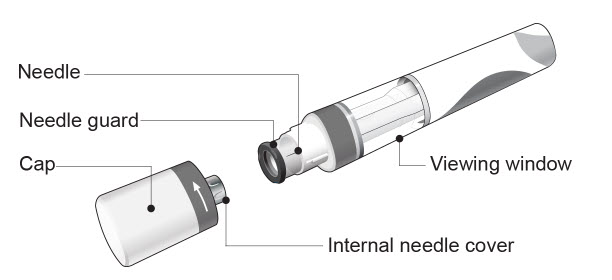 |
|
| The COSENTYX Sensoready Pen is shown above with the cap removed. Do not remove the cap until you are ready to inject. | |
| What you need for your injection: | |
| Included in the carton: A new COSENTYX Sensoready Pen (see Figure B). Each COSENTYX Sensoready Pen contains 150 mg of COSENTYX.
|
Figure B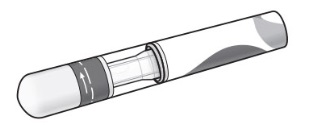 |
Not included in the carton (see Figure C):
See “How should I dispose of used COSENTYX Sensoready Pen?” at the end of this Instructions for Use. |
Figure C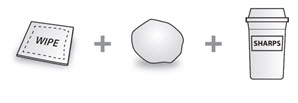 |
|
Before your injection: |
|
Step 1. Important safety checks before you inject (see Figure D):
Contact your pharmacist if the COSENTYX Sensoready Pen fails any of these checks. |
Figure D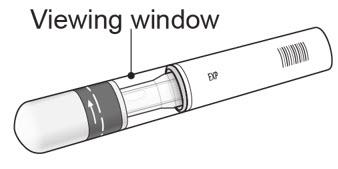 |
Step 2. Choose your injection site:
|
Figure E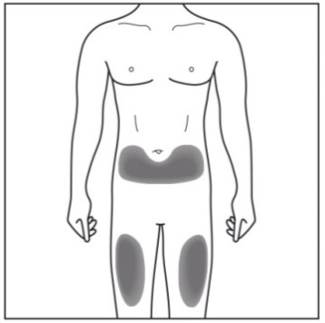 |
|
Figure F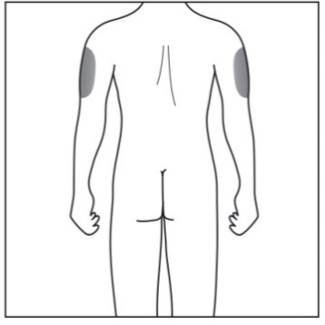 |
Step 3. Cleaning your injection site:
|
Figure G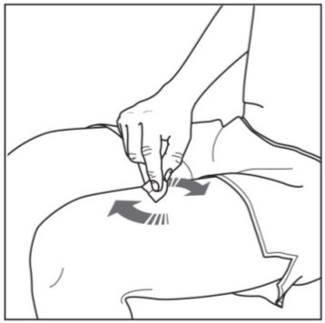 |
|
Your injection: |
|
Step 4. Removing the cap:
|
Figure H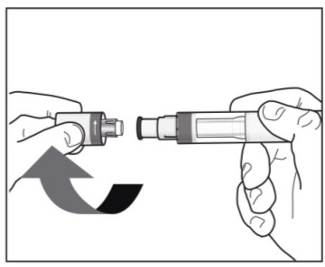 |
Step 5. Holding your COSENTYX Sensoready Pen:
|
Figure I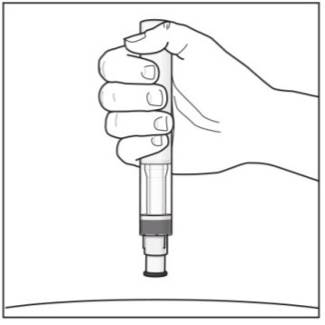 |
Important: During the injection you will hear 2 loud clicks:
You must keep holding the COSENTYX Sensoready Pen firmly against your skin until you see a green indicator fill the window and stop moving. |
|
Step 6. Starting your injection:
|
Figure J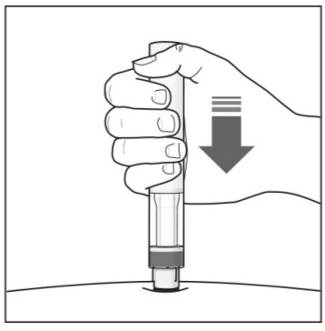 |
Step 7. Completing your injection:
|
Figure K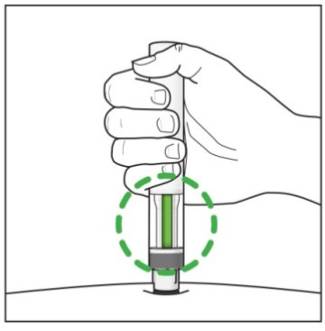 |
|
After your injection: |
|
Step 8. Check the green indicator fills the window (see Figure L):
If your prescribed dose of COSENTYX is 300 mg, repeat steps 1 through 8 with a new COSENTYX Sensoready Pen. |
Figure L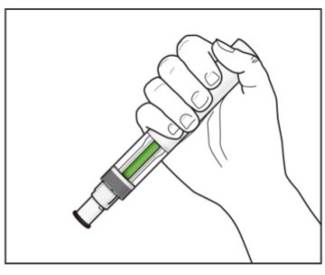 |
|
How should I dispose of used COSENTYX Sensoready Pens? |
|
| Step 9. Put your used Sensoready Pens in a FDA-cleared sharps disposal container right away after use (see Figure M). Do not throw away (dispose of) Sensoready Pens in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes, and Sensoready Pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL. |
Figure M |
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: December 2021 |
|
| Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 US License Number 1244 |
|
| © Novartis | |
| T2021-167 | |
SRC: NLM .