Corifact
Generic name: factor XIII
Brand names: Corifact, Tretten
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by A Ras MD.
What is Corifact?
Corifact is a prescription medicine that is used to treat or prevent bleeding.
Description
CORIFACT, Factor XIII Concentrate (Human), is a heat-treated, lyophilized concentrate of coagulation factor XIII for reconstitution for intravenous use. CORIFACT (FXIII) consists of two A-subunits and two B-subunits, and is made from pooled human plasma. Each vial contains 1000-1600 units FXIII, 120 to 200 mg human albumin, 120 to 320 mg total protein, 80 to 120 mg glucose and 140 to 220 mg sodium chloride. Sodium hydroxide may have been used to adjust the pH.
All plasma used in the manufacture of CORIFACT is obtained from US donors and is tested using serological assays for hepatitis B surface antigen and antibodies to HIV-1/2 and HCV. The plasma is tested with Nucleic Acid Testing (NAT) for HCV, HIV-1, HAV and HBV and found to be non-reactive (negative), and the plasma is also tested by NAT for Human Parvovirus B19. Only plasma that passed virus screening is used for production, and the limit for Parvovirus B19 in the fractionation pool is set not to exceed 104 International Units of Parvovirus B19 DNA per mL.
CORIFACT is manufactured from cryo-depleted plasma into an ethanol precipitate, which is then purified by the following four steps:
- Precipitation/adsorption
- Ion exchange chromatography
- Heat-treatment (+60°C for 10 hours in an aqueous solution)
- Virus filtration over two 20 nm filters in series
The sterile filtered final bulk solution is filled into vials and lyophilized. These four manufacturing steps were independently validated in a series of in vitro experiments for their capacity to inactivate or remove both enveloped and non-enveloped viruses. Table 4 shows the virus clearance capacity of the CORIFACT manufacturing process, expressed as mean log10 reduction factor.
| Manufacturing Steps | Virus Reduction Factor (log10) | |||||
|---|---|---|---|---|---|---|
| Enveloped Viruses | Non-Enveloped Viruses | |||||
| HIV | BVDV | WNV | PRV | HAV | CPV | |
| HIV, Human immunodeficiency virus type 1, model for HIV-1 and HIV-2 BVDV, bovine viral diarrhea virus, model for HCV WNV, West Nile virus PRV, pseudorabies virus, a model for large enveloped DNA viruses HAV, Hepatitis A virus CPV, canine parvovirus, model for B19V B19V, Human parvovirus B19 N/A, not applicable n.d., not done |
||||||
|
||||||
| Al(OH)3 Adsorption / Vitacel® and Defibrination | n.d. | n.d. | n.d. | 6.9 | n.d. | n.d. |
| Ion Exchange Chromatography | 5.0 | 3.3 | n.d. | ≥8.0 | 3.4 | 3.7 |
| Heat Treatment | ≥7.7 | ≥8.1 | ≥7.4 | N/A* | 4.3 | 1.0† |
| 20 nm / 20 nm Virus Filtration | ≥6.1 | ≥5.0 | ≥7.4 | ≥6.4 | ≥5.6 | 6.1 |
| Cumulative Virus Reduction (log10) | ≥18.8 | ≥16.4 | ≥14.8 | ≥21.3 | ≥13.3 | 10.8 |
Mechanism of Action
CORIFACT (FXIII) is an endogenous plasma glycoprotein consisting of two A-subunits and two B-subunits. FXIIIa promotes cross-linking of fibrin during coagulation and is essential to the physiological protection of the clot against fibrinolysis. FXIIIa is a transglutaminase enzyme that catalyzes the cross-linking of the fibrin α- and γ-chains for fibrin stabilization and renders the fibrin clot more elastic and resistant to fibrinolysis.2,3 FXIIIa also cross-links α2-plasmin inhibitor to the α-chain of fibrin, resulting in protection of the fibrin clot from degradation by plasmin. Cross-linked fibrin is the end result of the coagulation cascade, and provides tensile strength to a primary hemostatic platelet plug.3
The B-subunits in plasma have no enzymatic activity, and function as carrier molecules for the A-subunits. They stabilize the structure of the A-subunits and protect them from proteolysis.
Before taking Corifact, tell your doctor:
- If you are allergic to Corifact; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Corifact with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Corifact?
- Tell all of your health care providers that you take Corifact. This includes your doctors, nurses, pharmacists, and dentists.
- Allergic side effects may rarely happen.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Blood clots have happened with Corifact. Tell your doctor if you have ever had a blood clot. Talk with your doctor.
- Call the doctor right away if the normal dose does not work as well.
- This medicine is made from human plasma (part of the blood) and may have viruses that may cause disease. This medicine is screened, tested, and treated to lower the chance that it carries an infection. Talk with the doctor.
- Talk with the doctor before you travel. You will need to bring enough of Corifact for use during travel.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using Corifact while you are pregnant.
- Tell your doctor if you are breast-feeding. You will need to talk about any risks to your baby.
How is Corifact best taken?
Use Corifact as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into a vein.
- If you will be giving yourself the shot, your doctor or nurse will teach you how to give the shot.
- Wash your hands before and after use.
- If stored in a refrigerator, let Corifact come to room temperature before mixing. Do not heat Corifact.
- This medicine needs to be mixed before use. Follow how to mix as you were told by the doctor.
- Do not shake.
- Use within 4 hours of making.
- After mixing, do not refrigerate.
- Do not use if the solution is cloudy, leaking, or has particles.
- Do not use if solution changes color.
- Throw away needles in a needle/sharp disposal box. Do not reuse needles or other items. When the box is full, follow all local rules for getting rid of it. Talk with a doctor or pharmacist if you have any questions.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of Corifact that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of certain infections (parvovirus B19, hepatitis A) like fever or chills, feeling very sleepy, runny nose, rash, joint pain, tiredness, poor appetite, upset stomach or throwing up, belly pain, or yellow skin or eyes.
- Shortness of breath.
- Chest pain or pressure.
- Coughing up blood.
- Dizziness or passing out.
- Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight.
- Swelling, warmth, numbness, change of color, or pain in a leg or arm.
- Any unexplained bruising or bleeding.
- Fever or chills.
What are some other side effects of Corifact?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Headache.
- Joint pain.
- Irritation where the shot is given.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Corifact?
- Store in a refrigerator. Do not freeze.
- Store in the original container to protect from light.
- If needed, you may store at room temperature.
- Do not put Corifact back in the refrigerator after it has been stored at room temperature.
- If stored at room temperature, throw Corifact away after 6 months.
- If stored at room temperature, make a note of the date it was placed at room temperature.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
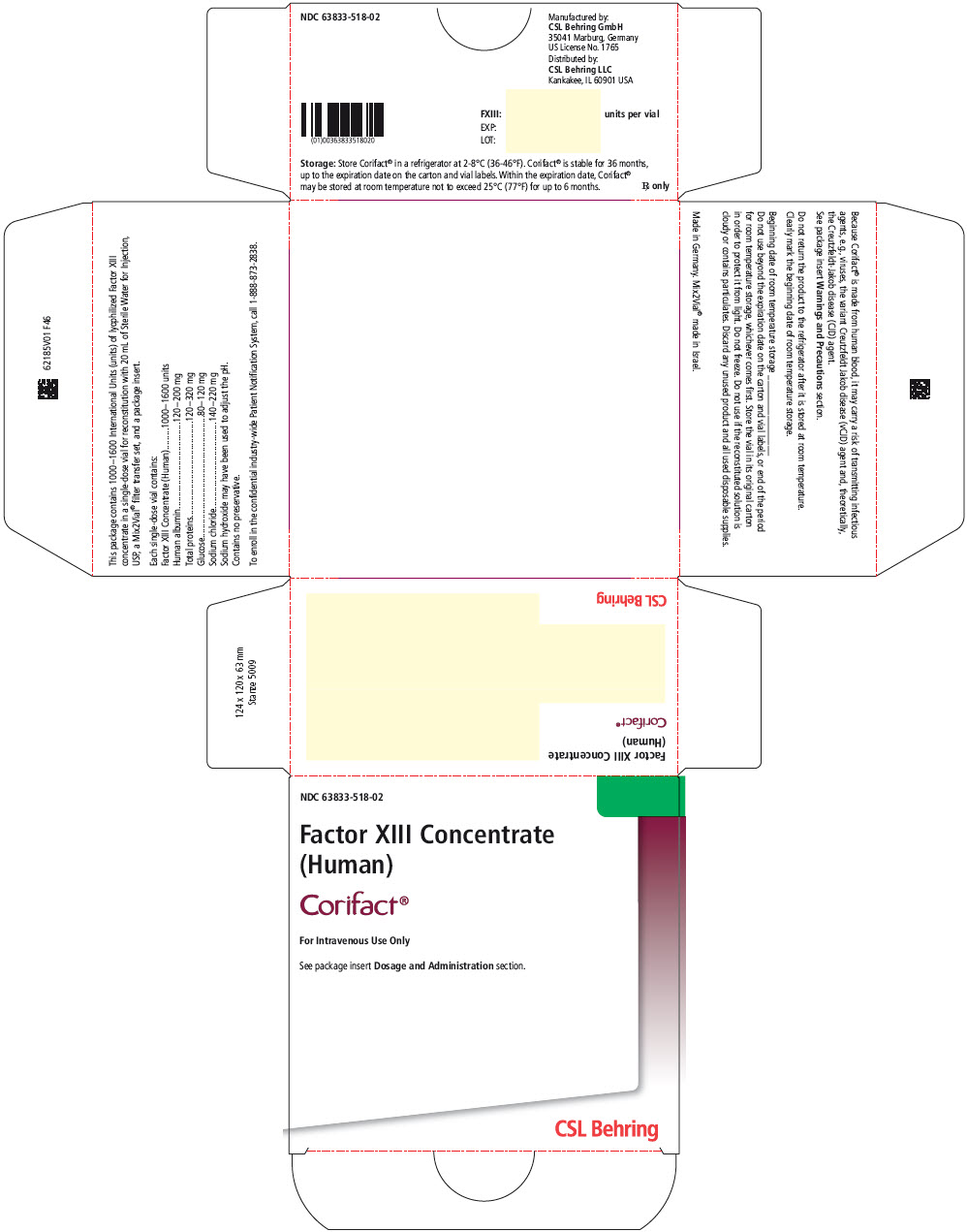
Label
PRINCIPAL DISPLAY PANEL – 1000-1600 IU VIAL LABEL
- Factor XIII Concentrate (Human)
- Corifact®
- For Intravenous Use Only
Rx only - Each Corifact® single-use vial contains 1000–1600
International Units (units) of lyophilized Factor XIII concentrate
for reconstitution with 20 mL of Sterile Water for Injection, USP.
Store Corifact® in a refrigerator at 2–8°C (36–46°F).
Corifact® is stable for 36 months, up to the expiration date on
the carton and vial labels. Do not use if the reconstituted
solution is cloudy or contains particulates. Do not freeze.
Protect from light. Contains no preservative.

PRINCIPAL DISPLAY PANEL – KIT CARTON
- NDC 63833-518-02
- Factor XIII Concentrate
(Human) - Corifact®
- For Intravenous Use Only
- See package insert Dosage and Administration section.
- CSL Behring
SRC: NLM .
