Cefoxitin
Generic name: cefoxitin
Brand name: Mefoxin
Dosage forms: injectable powder for injection (10 g); intravenous powder for injection (1 g; 2 g); intravenous solution (1 g/50 mL-iso-osmotic dextrose; 2 g/50 mL-iso-osmotic dextrose)
Drug class: Second generation cephalosporins
Medically reviewed by A Ras MD.
What is cefoxitin?
Cefoxitin is a prescription medicine that is used to treat or prevent bacterial infections.
Description
Cefoxitin for Injection and Dextrose Injection is a sterile, nonpyrogenic, single-dose packaged combination of cefoxitin sodium USP and Dextrose Injection (diluent) in the DUPLEX® sterile container. The DUPLEX® Container is a flexible dual chamber container.
The drug chamber is filled with cefoxitin sodium USP, a semi-synthetic, broad-spectrum cephalosporin antibacterial sealed under nitrogen for intravenous administration. Cefoxitin sodium USP is derived from cephamycin C, which is produced by Streptomyces lactamdurans. Its chemical name is sodium (6R,7S)-3-(hydroxymethyl)-7-methoxy-8-oxo-7-[2-(2-thienyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate carbamate (ester).
The empirical formula is C16H16N3NaO7S2, and the molecular weight is 449.44. The structural formula is:
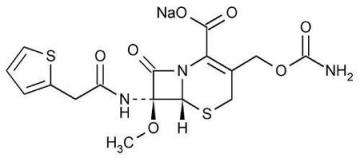
Cefoxitin sodium contains approximately 53.8 mg (2.3 mEq) of sodium per gram of cefoxitin activity.
The diluent chamber contains Dextrose Injection. The concentration of Dextrose Hydrous USP in Water for Injection USP has been adjusted to render the reconstituted drug product iso-osmotic. Dextrose Hydrous USP has been added to adjust the osmolality to approximately 290 mOsmol/kg (approximately 2 g (4% w/v) and 1.1 g (2.2% w/v) to the 1 g and 2 g doses, respectively). Dextrose Injection is sterile, nonpyrogenic, and contains no bacteriostatic and antimicrobial agents.
Dextrose Hydrous USP has the following structural (molecular) formula:
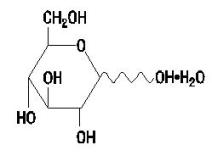
The molecular weight of Dextrose Hydrous USP is 198.17.
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use. When reconstituted according to instructions in the product labeling, the approximate osmolality of the reconstituted solution of Cefoxitin for Injection and Dextrose Injection is approximately 290 mOsmol/kg. After reconstitution, each 50 mL contains 1 gram of cefoxitin (equivalent to 1.05 gram of cefoxitin sodium) or 2 grams of cefoxitin (equivalent to 2.10 grams of cefoxitin sodium), with a pH of approximately 6.5. Solutions of Cefoxitin for Injection and Dextrose Injection range from colorless to light amber.
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
Before taking cefoxitin, tell your doctor:
- If you are allergic to cefoxitin; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have had stomach or bowel problems like colitis.
This is not a list of all drugs or health problems that interact with cefoxitin.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take cefoxitin with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take cefoxitin?
- Tell all of your health care providers that you take cefoxitin. This includes your doctors, nurses, pharmacists, and dentists.
- Severe and sometimes deadly allergic side effects have rarely happened with drugs like this one.
- Have your blood work checked if you are on cefoxitin for a long time. Talk with your doctor.
- If you have high blood sugar (diabetes) and test your urine glucose, talk with your doctor to find out which tests are best to use.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take cefoxitin.
- Do not use longer than you have been told. A second infection may happen.
- If you have myasthenia gravis, talk with your doctor. Call your doctor if your signs get worse. Signs of myasthenia gravis have also happened in people who do not have it. Call your doctor right away if you have new or worse muscle weakness, trouble chewing or swallowing, trouble breathing, droopy eyelids, or change in eyesight like blurred eyesight or seeing double.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is cefoxitin best taken?
Use cefoxitin as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as an infusion into a vein over a period of time.
- It may be given as a shot into a vein.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of cefoxitin that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of kidney problems like unable to pass urine, change in how much urine is passed, blood in the urine, or a big weight gain.
- Fever, chills, or sore throat; any unexplained bruising or bleeding; or feeling very tired or weak.
- Seizures.
- Flushing.
- Shortness of breath.
- Very bad dizziness or passing out.
- Dark urine or yellow skin or eyes.
- Diarrhea is common with antibiotics. Rarely, a severe form called C diff–associated diarrhea (CDAD) may happen. Sometimes, this has led to a deadly bowel problem (colitis). CDAD may happen during or a few months after taking antibiotics. Call your doctor right away if you have stomach pain, cramps, or very loose, watery, or bloody stools. Check with your doctor before treating diarrhea.
What are some other side effects of cefoxitin?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Diarrhea.
- Irritation where cefoxitin is given.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out cefoxitin?
- If you need to store cefoxitin at home, talk with your doctor, nurse, or pharmacist about how to store it.
Label
PRINCIPAL DISPLAY PANEL – 50 ML CONTAINER LABEL
- Cefoxitin for Injection
and Dextrose Injection - 1g*
- REF 3123-11
NDC 0264-3123-11 - DUPLEX® CONTAINER
- 50 mL
- Use only after mixing contents of both chambers.
For IV Use Only Iso-osmotic Single-Dose Sterile/Nonpyrogenic - * Contains cefoxitin sodium equivalent to 1 g cefoxitin.
- R
- LOT

SRC: NLM .
