Cefotetan
Generic name: cefotetan
Brand name: Cefotan
Dosage forms: injectable powder for injection (1 g; 2 g); intravenous solution (1 g/50 mL-iso-osmotic dextrose; 2 g/50 mL-iso-osmotic dextrose)
Drug class: Second generation cephalosporins
Medically reviewed by A Ras MD.
What is cefotetan?
Cefotetan is a prescription medicine that is used to treat or prevent bacterial infections.
Description
CEFOTAN (cefotetan disodium for injection) and CEFOTAN (cefotetan injection) in Galaxy® * plastic container (PL 2040) as cefotetan disodium are sterile, semisynthetic, broad-spectrum, beta-lactamase resistant, cephalosporin (cephamycin) antibiotics for parenteral administration. It is the disodium salt of [6R-(6α,7α)]-7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its molecular formula is
C17H15N7Na2O8S4 with a molecular weight of 619.57.
Structural Formula
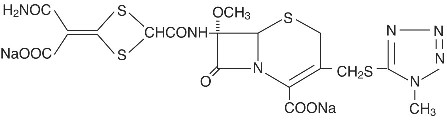
CEFOTAN (cefotetan disodium for injection) is supplied in vials containing 80 mg (3.5 mEq) of sodium per gram of cefotetan activity. It is a white to pale yellow powder which is very soluble in water. Reconstituted solutions of CEFOTAN (cefotetan disodium for injection) are intended for intravenous and intramuscular administration. The solution varies from colorless to yellow depending on the concentration. The pH of freshly reconstituted solutions is usually between 4.5 to 6.5.
CEFOTAN in the ADD-Vantage Vial† is intended for intravenous use only after dilution with the appropriate volume of ADD-Vantage diluent solution.
CEFOTAN is available in two vial strengths. Each CEFOTAN 1 g vial contains cefotetan disodium equivalent to 1 g cefotetan activity. Each CEFOTAN 2 g vial contains cefotetan disodium equivalent to 2 g cefotetan activity.
CEFOTAN (cefotetan injection) in the Galaxy® plastic container (PL 2040) is a frozen, iso-osmotic, sterile, nonpyrogenic premixed 50 mL solution containing 1 g or 2 g cefotetan as sterile cefotetan disodium. Dextrose, USP has been added to adjust the osmolality to 300 mOsmol/kg (approximately 1.9 g and 1.1 g to the 1 g and 2 g dosages, respectively); sodium bicarbonate has been added to convert cefotetan free acid to the sodium salt. The pH has been adjusted between 4 and 6.5 with sodium bicarbonate and may have been adjusted with hydrochloric acid. CEFOTAN (cefotetan injection) in the Galaxy® plastic container (PL 2040) contains 80 mg (3.5 mEq) of sodium per gram of cefotetan activity. After thawing to room temperature, the solution is intended for intravenous use only.
This Galaxy® container is fabricated from a specially designed multilayer plastic (PL 2040). Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration dating period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers as well as by tissue culture toxicity.
Before taking cefotetan, tell your doctor:
- If you are allergic to cefotetan; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If cefotetan or alike drugs caused low red blood cell counts before.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take cefotetan with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take cefotetan?
- Tell all of your health care providers that you take cefotetan. This includes your doctors, nurses, pharmacists, and dentists.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Avoid alcohol and products that have alcohol in them while taking cefotetan and for at least 72 hours after your last dose. Drinking alcohol or taking products that have alcohol in them, like some cough syrups, may cause flushing, sweating, headaches, and fast heartbeat.
- If you have high blood sugar (diabetes) and test your urine glucose, talk with your doctor to find out which tests are best to use.
- Do not use longer than you have been told. A second infection may happen.
- This medicine may affect certain lab tests. Tell all of your health care providers and lab workers that you take cefotetan.
- If you are 65 or older, use cefotetan with care. You could have more side effects.
- Tell your doctor if you are pregnant, plan on getting pregnant, or are breast-feeding. You will need to talk about the benefits and risks to you and the baby.
How is cefotetan best taken?
Use cefotetan as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into a muscle or as an infusion into a vein over a period of time.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of cefotetan that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Any unexplained bruising or bleeding.
- Not able to pass urine or change in how much urine is passed.
- Fever or chills.
- Sore throat.
- Seizures.
- Vaginal itching or discharge.
- Diarrhea is common with antibiotics. Rarely, a severe form called C diff–associated diarrhea (CDAD) may happen. Sometimes, this has led to a deadly bowel problem (colitis). CDAD may happen during or a few months after taking antibiotics. Call your doctor right away if you have stomach pain, cramps, or very loose, watery, or bloody stools. Check with your doctor before treating diarrhea.
- Rarely, a very bad blood problem called hemolytic anemia has happened with cefotetan. Sometimes it has been deadly. The chance of getting hemolytic anemia may be higher with cefotetan than with other drugs like this one. Call your doctor right away if you feel very tired or weak, or if you develop dark urine or yellow skin or eyes while you are using cefotetan or within 3 weeks after you stop it.
What are some other side effects of cefotetan?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Diarrhea.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out cefotetan?
- If you need to store cefotetan at home, talk with your doctor, nurse, or pharmacist about how to store it.
Label
PACKAGE LABEL – PRINCIPAL DISPLAY – Cefotetan 1 gram Vial Label
- NDC 63323-385-01
- 308510
- CEFOTETAN
FOR INJECTION, USP
1 gram per vial* - For IM or IV Use
- Rx only

PACKAGE LABEL – PRINCIPAL DISPLAY – Cefotetan 1 gram Vial Carton Panel
- NDC 63323-385-10
- 308510
- CEFOTETAN
FOR INJECTION, USP - 1 gram per vial*
- For Intramuscular or Intravenous Use
Rx only - 10 x 1 gram Vials

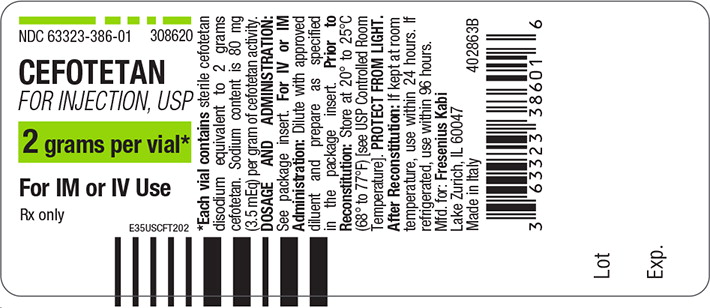
PACKAGE LABEL – PRINCIPAL DISPLAY – Cefotetan 2 grams Vial Label
- NDC 63323-386-01
- 308620
- CEFOTETAN
FOR INJECTION, USP - 2 grams per vial*
- For IM or IV Use
- Rx only
PACKAGE LABEL – PRINCIPAL DISPLAY – Cefotetan 2 grams Vial Carton Panel
- NDC 63323-386-20
- 308620
- CEFOTETAN
FOR INJECTION, USP - 2 grams per vial*
- For Intramuscular or Intravenous Use
Rx only - 10 x 2 gram Vials

SRC: NLM .
