Cardura XL
Generic name: doxazosin
Brand names: Cardura, Cardura XL
Drug classes: Alpha-adrenoreceptor antagonists, Antiadrenergic agents, peripherally acting
Medically reviewed by A Ras MD.
What is Cardura XL?
Cardura XL is a prescription medicine called an “alpha-blocker.” Cardura XL is used to treat the symptoms of benign prostatic hyperplasia (BPH). Cardura XL may help to relax the muscles in the prostate and the bladder which may lessen the symptoms of BPH and improve urine flow.
Before prescribing Cardura XL, your healthcare provider may examine your prostate gland and do a blood test called a prostate specific antigen (PSA) test to check for prostate cancer. Prostate cancer and BPH can cause the same symptoms. Prostate cancer needs a different treatment.
Some medicines called “alpha-blockers” are used to treat high blood pressure. Cardura XL is not for the treatment of high blood pressure.
It is not known if Cardura XL is safe and effective in children.
Description
CARDURA XL contains doxazosin which is a quinazoline compound with the chemical name 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. The empirical formula for doxazosin mesylate is C23H25N5O5 ∙ CH4O3S and the molecular weight is 547.6. It has the following structure:
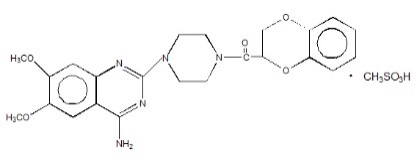
CARDURA XL is an extended release tablet for oral use and is designed to deliver 4 or 8 mg of doxazosin as the free base. Each 4 and 8 mg tablet contains 5.1 and 10.2 mg doxazosin mesylate (includes a 5% overage) to ensure the labeled dose of 4 and 8 mg doxazosin as the free base (equivalent to 4.9 and 9.8 mg of doxazosin mesylate), is delivered. The inactive ingredients for CARDURA XL are: polyethylene oxide, sodium chloride, hypromellose, red ferric oxide, titanium dioxide, magnesium stearate, cellulose acetate, Macrogol®, pharmaceutical glaze, and black iron oxide.
CARDURA XL System Components and Performance
CARDURA XL is similar in appearance to a conventional tablet. It consists, however, of an osmotically active drug core surrounded by a semipermeable membrane. The core itself is divided into two layers: an “active” layer containing the drug, and a “push” layer containing pharmacologically inert (but osmotically active) components. The membrane surrounding the tablet is permeable to water, but not to drug or osmotic excipients. As water from the gastrointestinal tract enters the tablet, pressure increases in the osmotic layer and “pushes” against the drug layer, resulting in the release of drug through a small, laser-drilled orifice in the membrane on the drug side of the tablet.
CARDURA XL utilizes GITS (Gastrointestinal Therapeutic System) which is designed to provide a controlled rate of delivery of doxazosin into the gastrointestinal lumen which is independent of pH or gastrointestinal (GI) motility. The function of CARDURA XL depends upon the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the GI tract. Drug delivery is essentially constant as long as the osmotic gradient remains constant, and then gradually falls to zero. The biologically inert components of the tablet remain intact during GI transit and are eliminated in the feces as an insoluble shell.
Mechanism of Action
The symptoms associated with benign prostatic hyperplasia (BPH), such as urinary frequency, nocturia, weak stream, hesitancy, and incomplete emptying are related to two components, anatomical (static) and functional (dynamic). The static component is related to an increase in prostate size caused, in part, by a proliferation of smooth muscle cells in the prostatic stroma. However, the severity of BPH symptoms and the degree of urethral obstruction do not correlate well with the size of the prostate.
The dynamic component of BPH is associated with an increase in smooth muscle tone in the prostate and bladder neck. The degree of tone in this area is mediated by the alpha1 adrenoceptor, which is present in high density in the prostatic stroma, prostatic capsule, and bladder neck. Blockade of the alpha1 receptor decreases urethral resistance and may relieve the BPH symptoms and improve urine flow. Doxazosin mesylate is a selective inhibitor of the alpha1-subtype of alpha adrenergic receptors. In the human prostate, doxazosin mesylate antagonizes phenylephrine (alpha1 agonist)-induced contractions, in vitro, and binds with high affinity to the alpha1A adrenoceptor.
Who should not take Cardura XL?
Do not take Cardura XL if you are allergic to doxazosin, other medications called quinazolones, or any of the ingredients in Cardura XL. See the end of this guide for a complete list of ingredients in Cardura XL.
What should I tell my healthcare provider before taking Cardura XL?
Before you take Cardura XL, tell your healthcare provider if you:
- have or have had low blood pressure, especially after taking another medicine. Signs of low blood pressure are fainting, dizziness, and lightheadedness.
- plan to have cataract surgery
- have stomach problems
- have prostate cancer
- have liver problems
- have heart problems
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Cardura XL may affect the way other medicines work, and other medicines may affect how Cardura XL works.
Especially tell your healthcare provider if you take:
- a medicine to treat an infection
- a medicine to treat HIV
- a medicine to treat depression
- a medicine to treat erectile dysfunction (ED)
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one of those listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Cardura XL?
- Take Cardura XL exactly as your healthcare provider tells you to take it.
- Take Cardura XL 1 time each day with your breakfast.
- Take Cardura XL tablets whole. Do not chew, divide, cut, or crush Cardura XL tablets before swallowing. If you cannot swallow Cardura XL tablets whole, tell your healthcare provider. You may need a different medicine.
- Cardura XL tablets have an outside shell that helps to release the medicine inside over time. You may see the empty Cardura XL tablet in your stool (bowel movement). This is normal.
- If you take too much Cardura XL, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Cardura XL?
Do not drive, operate machinery, or do other dangerous activities until you know how Cardura XL affects you. Cardura XL can cause dizziness, weakness, or fainting.
What are the possible side effects of Cardura XL?
Cardura XL may cause serious side effects including:
- a sudden drop in blood pressure (postural hypotension). Postural hypotension can happen at any time while you take Cardura XL. The chance of having postural hypotension is higher a few hours after you take Cardura XL or if you start taking a higher dose.
Symptoms of postural hypotension may happen after you stand up from a lying or sitting position and may include:- dizziness or
- fainting or
- feeling light-headed
You should get up slowly from a chair or bed until you know how Cardura XL will affect you. If you begin to feel dizzy or lightheaded, sit or lie down with your legs and feet up. If your symptoms do not get better call your healthcare provider.
- intraoperative floppy iris syndrome (IFIS). IFIS can happen during eye surgery to people who are taking or have taken alpha-blockers such as Cardura XL. If you have an eye surgery for a clouding of the eye (cataract) planned, tell your eye doctor that you are using Cardura XL or have taken an alpha-blocker before.
- blockage of the digestive tract. There have been rare reports of symptoms of blockage in the digestive tract in patients with prior history of narrowing or blockage of the digestive tract. These symptoms are severe and persistent abdominal pain/cramping/bloating. If you know you have a narrowing of your digestive tract and you experience these symptoms, contact your healthcare provider.
- prolonged erection of the penis. Extremely rarely, Cardura XL and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
The most common side effects of Cardura XL include:
- tiredness
- headache
- low blood pressure
- dizziness
Call your healthcare provider if you get any side effect that bothers you or that does not go away.
These are not all the possible side effects of Cardura XL. For more information ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Cardura XL
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cardura XL for a condition for which it was not prescribed. Do not give Cardura XL to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information guide summarizes the most important information about Cardura XL. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Cardura XL that is written for health professionals.
For more information, call 1-800-438-1985.
How should I store Cardura XL?
- Store Cardura XL at 59°F to 86°F (15°C to 30°C).
Keep Cardura XL and all medicines out of reach of children.
What are the ingredients in Cardura XL?
Active Ingredient: doxazosin mesylate
Inactive Ingredients: polyethylene oxide, sodium chloride, hypromellose, red ferric oxide, titanium dioxide, magnesium stearate, cellulose acetate, Macrogol, pharmaceutical glaze, and black iron oxide.
Label
PRINCIPAL DISPLAY PANEL – 8 MG TABLET BOTTLE LABEL
- Pfizer
NDC 0049-2720-30 - Cardura® XL
(doxazosin mesylate
extended release tablets) - 8 mg*
- 30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL – 4 MG TABLET BOTTLE LABEL – 2040
- Pfizer
NDC 0049-2040-10 - Cardura® XL
(doxazosin)
extended release tablets - 4 mg*
- 30 Tablets
Rx only


SRC: NLM .

