Brukinsa
Generic name: zanubrutinib
Drug class: BTK inhibitors
Medically reviewed by A Ras MD.
What is Brukinsa?
Brukinsa is a prescription medicine used to treat adults with mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer.
It is not known if Brukinsa is safe and effective in children.
Description
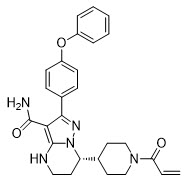
BRUKINSA (zanubrutinib) is a kinase inhibitor. The empirical formula of zanubrutinib is C27H29N5O3 and the chemical name is (S)-7-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide. Zanubrutinib is a white to off-white powder, with a pH of 7.8 in saturated solution. The aqueous solubility of zanubrutinib is pH dependent, from very slightly soluble to practically insoluble.
The molecular weight of zanubrutinib is 471.55 Daltons.
Zanubrutinib has the following structure:
Each BRUKINSA capsule for oral administration contains 80 mg zanubrutinib and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose and sodium lauryl sulfate. The capsule shell contains edible black ink, gelatin, and titanium dioxide.
Mechanism of Action
Zanubrutinib is a small-molecule inhibitor of Bruton’s tyrosine kinase (BTK). Zanubrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. In B-cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis and adhesion. In nonclinical studies, zanubrutinib inhibited malignant B-cell proliferation and reduced tumor growth.
What should I tell my healthcare provider before taking Brukinsa?
Before taking Brukinsa, tell your healthcare provider about all of your medical conditions, including if you:
- have bleeding problems.
- have had recent surgery or plan to have surgery. Your healthcare provider may stop Brukinsa for any planned medical, surgical, or dental procedure.
- have an infection.
- have or had heart rhythm problems.
- have high blood pressure.
- have liver problems, including a history of hepatitis B virus (HBV) infection.
- are pregnant or plan to become pregnant. Brukinsa can harm your unborn baby. If you are able to become pregnant, your healthcare provider may do a pregnancy test before starting treatment with Brukinsa.
- Females should not become pregnant during treatment and for at least 1 week after the last dose of Brukinsa. You should use effective birth control (contraception) during treatment and for at least 1 week after the last dose of Brukinsa.
- Males should avoid getting female partners pregnant during treatment and for at least 1 week after the last dose of Brukinsa. You should use effective birth control (contraception) during treatment and for at least 1 week after the last dose of Brukinsa.
- are breastfeeding or plan to breastfeed. It is not known if Brukinsa passes into your breast milk. Do not breastfeed during treatment with Brukinsa and for at least 2 weeks after your last dose of Brukinsa.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Brukinsa with certain other medications may affect how Brukinsa works and can cause side effects.
How should I take Brukinsa?
- Take Brukinsa exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking Brukinsa unless your healthcare provider tells you to.
- Your healthcare provider may tell you to decrease your dose, temporarily stop, or completely stop taking Brukinsa if you develop certain side effects.
- Take Brukinsa with or without food.
- Swallow Brukinsa capsules whole with a glass of water. Do not open, break, or chew the capsules.
- If you miss a dose of Brukinsa, take it as soon as you remember on the same day. Return to your normal schedule the next day.
What are the possible side effects of Brukinsa?
Brukinsa may cause serious side effects, including:
- Bleeding problems (hemorrhage) that can be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs or symptoms of bleeding, including:
- blood in your stools or black stools (looks like tar)
- pink or brown urine
- unexpected bleeding, or bleeding that is severe or you cannot control
- vomit blood or vomit that looks like coffee grounds
- cough up blood or blood clots
- increased bruising
- dizziness
- weakness
- confusion
- changes in speech
- headache that lasts a long time
- Infections that can be serious and may lead to death. Tell your healthcare provider right away if you have fever, chills, or flu-like symptoms.
- Decrease in blood cell counts. Decreased blood counts (white blood cells, platelets, and red blood cells) are common with Brukinsa, but can also be severe. Your healthcare provider should do blood tests during treatment with Brukinsa to check your blood counts.
- Second primary cancers. New cancers have happened in people during treatment with Brukinsa, including cancers of the skin. Use sun protection when you are outside in sunlight.
- Heart rhythm problems (atrial fibrillation and atrial flutter). Tell your healthcare provider if you have any of the following signs or symptoms:
- your heartbeat is fast or irregular
- feel lightheaded or dizzy
- pass out (faint)
- shortness of breath
- chest discomfort
The most common side effects of Brukinsa include:
- decreased white blood cells
- decreased platelet count
- rash
- diarrhea
- upper respiratory infection
- decreased red blood cells (anemia)
- bruising
- cough
These are not all the possible side effects of Brukinsa.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Brukinsa
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Brukinsa for a condition for which it was not prescribed. Do not give Brukinsa to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about Brukinsa that is written for healthcare professionals.
How should I store Brukinsa?
- Store Brukinsa capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Brukinsa comes in a bottle with a child-resistant cap.
Keep Brukinsa and all medicines out of the reach of children.
What are the ingredients in Brukinsa?
Active ingredient: zanubrutinib
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
Capsule shell contains edible black ink, gelatin, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 80 MG CAPSULE CARTON
- NDC 72579-011-02
Rx only - Brukinsa®
zanubrutinib
capsules - 80 mg
- Do not open, break or
chew the capsules - 120 Capsules
![]()
SRC: NLM .
