Braftovi
Generic name: encorafenib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Braftovi?
Braftovi is a prescription medicine that is used to treat a type of skin cancer (melanoma).
Description
Encorafenib is a kinase inhibitor. The chemical name is methyl N-{(2S)-1-[(4-{3-[5-chloro-2-fluoro-3-(methanesulfonamido)phenyl]-1-(propan-2-yl)-1H-pyrazol-4-yl}pyrimidin-2-yl)amino]propan-2-yl} carbamate. The molecular formula is C22H27ClFN7O4S and the molecular weight is 540 daltons. The chemical structure of encorafenib is shown below:
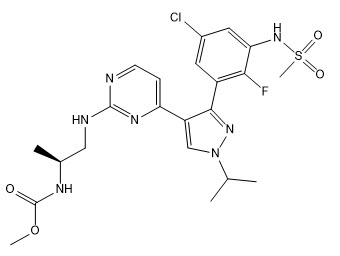
Encorafenib is a white to almost white powder. In aqueous media, encorafenib is slightly soluble at pH 1, very slightly soluble at pH 2, and insoluble at pH 3 and higher.
BRAFTOVI (encorafenib) capsules for oral use contain 75 mg of encorafenib with the following inactive ingredients: copovidone, poloxamer 188, microcrystalline cellulose, succinic acid, crospovidone, colloidal silicon dioxide, magnesium stearate (vegetable origin). The capsule shell contains gelatin, titanium dioxide, iron oxide red, iron oxide yellow, ferrosoferric oxide, monogramming ink (pharmaceutical glaze, ferrosoferric oxide, propylene glycol).
Mechanism of Action
Encorafenib is a kinase inhibitor that targets BRAF V600E, as well as wild-type BRAF and CRAF in in vitro cell-free assays with IC50 values of 0.35, 0.47, and 0.3 nM, respectively. Mutations in the BRAF gene, such as BRAF V600E, can result in constitutively activated BRAF kinases that may stimulate tumor cell growth. Encorafenib was also able to bind to other kinases in vitro including JNK1, JNK2, JNK3, LIMK1, LIMK2, MEK4, and STK36 and reduce ligand binding to these kinases at clinically achievable concentrations (≤0.9 µM).
Encorafenib inhibited in vitro growth of tumor cell lines expressing BRAF V600 E, D, and K mutations. In mice implanted with tumor cells expressing BRAF V600E, encorafenib induced tumor regressions associated with RAF/MEK/ERK pathway suppression.
Encorafenib and binimetinib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared with either drug alone, coadministration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of encorafenib and binimetinib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone.
In the setting of BRAF-mutant CRC, induction of EGFR-mediated MAPK pathway activation has been identified as a mechanism of resistance to BRAF inhibitors. Combinations of a BRAF inhibitor and agents targeting EGFR have been shown to overcome this resistance mechanism in nonclinical models. Coadministration of encorafenib and cetuximab had an anti-tumor effect greater than either drug alone, in a mouse model of colorectal cancer with mutated BRAF V600E.
Before taking Braftovi, tell your doctor:
- If you are allergic to Braftovi; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have any of these health problems: Low potassium or magnesium levels.
- If you take any other drugs (prescription or OTC, natural products, vitamins). There are many drugs that interact with Braftovi, like certain drugs that are used for HIV, infections, or seizures.
- If you are breast-feeding. Do not breast-feed while you take Braftovi and for 2 weeks after your last dose.
This is not a list of all drugs or health problems that interact with this medicine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Braftovi with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Braftovi?
- Tell all of your health care providers that you take Braftovi. This includes your doctors, nurses, pharmacists, and dentists.
- Encorafenib is taken with binimetinib. Be sure you know about the warnings, benefits, and risks of each drug. Talk with your doctor if you have questions or concerns about these drugs.
- Very bad bleeding problems have happened when encorafenib was taken with binimetinib. This includes bleeding in the stomach or brain. Sometimes, these bleeding problems have been deadly. If you have any questions, talk with your doctor.
- This medicine may add to the chance of getting some types of cancer. Talk with the doctor.
- Have your skin checked. Tell your doctor if you have any skin changes like a new wart, skin sore or reddish bump that bleeds or does not heal, or a change in the color or size of a mole.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Have an eye exam as you have been told by your doctor.
- If you have high blood sugar (diabetes), talk with your doctor. This medicine may raise blood sugar.
- Check your blood sugar as you have been told by your doctor.
- Avoid grapefruit and grapefruit juice.
- This medicine may affect being able to father a child. Talk with the doctor.
- This medicine may cause harm to an unborn baby. A pregnancy test will be done before you start Braftovi to show that you are NOT pregnant.
- Use a non-hormone type of birth control like condoms to prevent pregnancy while taking Braftovi and for at least 2 weeks after stopping Braftovi. Birth control pills and other hormone-based birth control may not work as well to prevent pregnancy.
- If you get pregnant while taking Braftovi or within 2 weeks after your last dose, call your doctor right away.
How is Braftovi best taken?
Use Braftovi as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take with or without food.
- Keep taking Braftovi as you have been told by your doctor or other health care provider, even if you feel well.
- If you throw up after taking a dose, do not repeat the dose. Take your next dose at your normal time.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is less than 12 hours until the next dose, skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of Braftovi that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of bleeding like throwing up or coughing up blood; vomit that looks like coffee grounds; blood in the urine; black, red, or tarry stools; bleeding from the gums; abnormal vaginal bleeding; bruises without a cause or that get bigger; or bleeding you cannot stop.
- Signs of electrolyte problems like mood changes, confusion, muscle pain or weakness, a heartbeat that does not feel normal, seizures, not hungry, or very bad upset stomach or throwing up.
- Signs of high blood sugar like confusion, feeling sleepy, more thirst, more hungry, passing urine more often, flushing, fast breathing, or breath that smells like fruit.
- Fever or chills.
- Change in eyesight, eye pain, or very bad eye irritation.
- Seeing colored dots.
- Seeing halos or bright colors around lights.
- Very bad headache.
- Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight.
- A burning, numbness, or tingling feeling that is not normal.
- Redness or irritation of the palms of hands or soles of feet.
- Feeling very tired or weak.
- A type of abnormal heartbeat (prolonged QT interval) can happen with Braftovi. Call your doctor right away if you have a fast heartbeat, a heartbeat that does not feel normal, or if you pass out.
What are some other side effects of Braftovi?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Feeling dizzy, tired, or weak.
- Upset stomach or throwing up.
- Stomach pain.
- Back, muscle, or joint pain.
- Constipation.
- Pain in arms or legs.
- Change in skin to hard and thick.
- Dry skin.
- Pimples (acne).
- Hair loss.
- Headache.
- Change in taste.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Braftovi?
- Store at room temperature.
- Store in the original container. Do not take out the antimoisture cube or packet.
- Keep lid tightly closed.
- Store in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
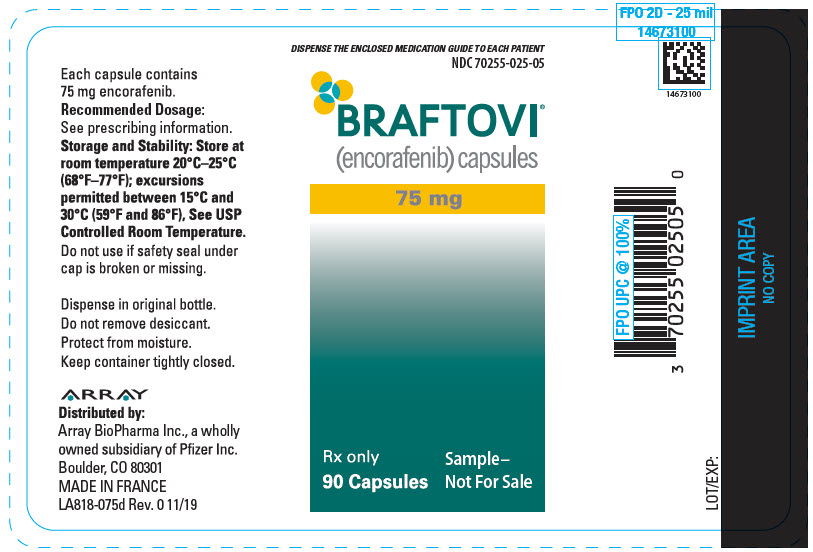
Label
PRINCIPAL DISPLAY PANEL – 75 MG CAPSULE BOTTLE LABEL – 025-05
- DISPENSE THE ENCLOSED MEDICATION GUIDE TO EACH PATIENT
- NDC 70255-025-05
- BRAFTOVI®
(encorafenib) capsules - 75 mg
- Rx only
90 Capsules - Sample–
Not For Sale
SRC: NLM .
