Beconase AQ
Generic name: beclomethasone nasal
Brand names: Beconase AQ, Qnasl
Drug class: Nasal steroids
Medically reviewed by A Ras MD.
What is Beconase AQ?
Beconase AQ is an anti-inflammatory nasal spray used for relieve the symptoms of seasonal or perennial allergic and nonallergic (vasomotor) rhinitis.
Description
Beclomethasone dipropionate, monohydrate, the active component of BECONASE AQ Nasal Spray, is an anti-inflammatory steroid having the chemical name 9-chloro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, monohydrate and the following chemical structure:
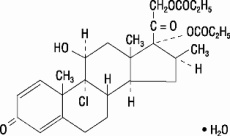
Beclomethasone 17,21-dipropionate is a diester of beclomethasone, a synthetic halogenated corticosteroid. Beclomethasone dipropionate, monohydrate is a white to creamy-white, odorless powder with a molecular weight of 539.06. It is very slightly soluble in water, very soluble in chloroform, and freely soluble in acetone and in ethanol.
BECONASE AQ Nasal Spray is a metered-dose, manual pump spray unit containing a microcrystalline suspension of beclomethasone dipropionate, monohydrate equivalent to 42 mcg of beclomethasone dipropionate, calculated on the dried basis, in an aqueous medium containing microcrystalline cellulose, carboxymethylcellulose sodium, dextrose, benzalkonium chloride, polysorbate 80, and 0.25% v/w phenylethyl alcohol. The pH through expiry is 5.0 to 6.8.
After initial priming (6 actuations), each actuation of the pump delivers from the nasal adapter 100 mg of suspension containing beclomethasone dipropionate, monohydrate equivalent to 42 mcg of beclomethasone dipropionate. If the pump is not used for 7 days, it should be primed until a fine spray appears. Each 25-g bottle of BECONASE AQ Nasal Spray provides 180 metered sprays.
Mechanism of Action:
Following topical administration, beclomethasone dipropionate produces anti-inflammatory and vasoconstrictor effects. The mechanisms responsible for the anti-inflammatory action of beclomethasone dipropionate are unknown. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. The direct relationship of these findings to the effects of beclomethasone dipropionate on allergic rhinitis symptoms is not known.
Biopsies of nasal mucosa obtained during clinical studies showed no histopathologic changes when beclomethasone dipropionate was administered intranasally.
Beclomethasone dipropionate is a pro-drug with weak glucocorticoid receptor binding affinity. It is hydrolyzed via esterase enzymes to its active metabolite beclomethasone-17-monopropionate (B-17-MP), which has high topical anti-inflammatory activity.
What is the most important information I should know about Beconase AQ?
For intranasal use only. Shake well before use.
Caution: Beconase AQ Nasal Spray is not intended to give rapid relief of your nasal symptoms. Beconase AQ Nasal Spray controls the underlying disorders responsible for your attacks, so it is important that you use it regularly at the times recommended by your doctor. The full benefit of Beconase AQ Nasal Spray may take a few days to develop.
How should I take Beconase AQ?
See Instructions for use below.
How should I store Beconase AQ?
Store between 15° and 30°C (59° and 86°F).
What are the ingredients in Beconase AQ?
Active ingredient: beclomethasone dipropionate monohydrate
Inactive ingredients: microcrystalline cellulose; carboxymethylcellulose sodium, unspecified form; dextrose, unspecified form; benzalkonium chloride; polysorbate 80; phenylethyl alcohol.
Instructions for use
Shake the suspension spray bottle well before using it. Read complete instructions carefully and use only as directed.
To Use:
1. Remove the safety clip and the plastic dust cap from the nasal applicator (Figure 1).
Figure 1
2. The very first time the spray is used, prime the pump into the air by pressing downward on the white collar, using your forefinger and middle finger while supporting the base of the bottle with your thumb. When you prime the pump for the first time, press down and release the pump 6 times or until a fine spray appears (Figure 2).
The pump is now ready for use. If the pump is not used for 7 days, prime until a fine spray appears.
Figure 2
3. Gently blow your nose to clear your nostrils. Close 1 nostril. Tilt your head forward slightly and, keeping the bottle upright, carefully insert the nasal applicator into the other nostril (Figure 3).
Figure 3
4. For each spray, press firmly downward once on the white collar, using your forefinger and middle finger while supporting the base of the bottle with your thumb. Avoid spraying in eyes. Breathe gently inward through the nostril.
5. Breathe out through your mouth.
6. Repeat steps 5 through 7 in the other nostril.
7. Replace the plastic dust cap and safety clip.
8. Discard the bottle after the date calculated by your doctor or pharmacist. The correct amount of medication in each spray cannot be assured after 180 sprays even though the bottle is not completely empty. Discard the bottle after 180 sprays. Before the discard date you should consult your doctor to see if a refill is needed. Do not take extra doses or stop taking Beconase AQ Nasal Spray without consulting your doctor.
Cleansing: To clean the nasal applicator, remove the plastic dust cap and safety clip and then press gently upward on the white collar to free the nasal applicator. Wash the applicator and dust cap with cold water. Dry and replace with the plastic dust cap and safety clip back in position.
If the nasal applicator becomes blocked, remove the dust cap, unscrew the complete pump mechanism, and soak the pump in warm water for a few minutes. Rinse with cold water, dry, refit to bottle, and reprime the pump.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0388-79
- Beconase AQ
- (beclomethasone dipropionate, monohydrate)
- Nasal Spray, 42 mcg
- 25 g
- 180 Metered Sprays
- Spray-For Intranasal Use Only
- Rx only
- Important: Read accompanying directions carefully.
- gsk
- Product of Italy
- ©2022 GSK group of companies or its licensor.
- 30000000000329 Rev. 4/22

SRC: NLM .
